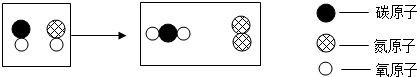
��Ŀ����
�ڡ����-��-���š�֮�佨����ϵ���ǻ�ѧѧ�����е�˼ά��ʽ��ͼ�� ��ʾ̼ԭ�ӡ�
��ʾ̼ԭ�ӡ� ��ʾ��ԭ�ӡ�
��ʾ��ԭ�ӡ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ�
��1��ˮú����һ����Ҫ������ȼ�ϣ�����������ˮú����Ӧ���۹���ʾ��ͼ��

�ټס��ҡ����������������������______������ţ���
�ڼ��У��⡢��Ԫ�ص�������Ϊ______��
�۸÷�Ӧ�Ļ�����Ӧ����Ϊ______��
��2����һ�ܱ���������A��B�������ʸ�96g��ɵĻ�����һ�������·�����ѧ��Ӧ����Ӧ����������88g C��36g D������B��A��C��D�������ʵ���ʾ��ͼ���±���ʾ��
| ���� | A | C | D |
| ��ʾ��ͼ |  |  |  |
����֪��B����Է�������Ϊ28���벹ȫ�÷�Ӧ�Ļ�ѧ����ʽ��______O2+______�T2CO2+2H2O��
�⣺��1����ͼʾ���Կ����÷�Ӧ�ķ�Ӧ����ˮ��̼����������һ����̼����������Ӧ�ķ���ʽΪH2O+C CO+H2��
CO+H2��
���������Ǻ�������Ԫ��������һ������Ԫ�صĴ�����ʼס��ҡ���������������������Ǽͱ���
�ڼĻ�ѧʽΪH2O�����������Ԫ�ص���������1��2��16=1��8��
�۸÷�Ӧ�ķ�Ӧ�����������һ�ֵ��ʺ�һ�ֻ�����ʸ÷�Ӧ���û���Ӧ��
��2��������Ŀ��������֪�÷�Ӧ��������CD������֮����88g+36g=124g����96g����B�Ƿ�Ӧ����������غ㶨�ɿ�֪�μӷ�Ӧ��B��������124g-96g=28g����������ʣ���B��������96g-28g=68g��
�ڸ��ݸ÷�Ӧ�ķ���ʽ�����֪��Ӧ��Bһ������̼������Ԫ�أ����ܺ�����Ԫ�أ�B����Է�������Ϊ28�����Կ�֪��������ֻ����̼������Ԫ�أ���������ʵĻ�ѧʽΪCxHy�����ڷ�Ӧǰ�����ԭ�ӵĸ�������ȣ���������ǰ�Ļ�ѧ������Ϊ3�����ڷ�Ӧ���������е�̼������Ԫ�ؾ�������CxHy�����Ը�������̼Ԫ�ص�����Ϊ88g��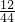 =24g����Ԫ�ص�����Ϊ36g��
=24g����Ԫ�ص�����Ϊ36g��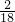 =4g������
=4g������
CxHy��̼��Ԫ�ص�����֮��Ϊ ��
�� ���ʻ�ѧʽΪC2H4�������ʵ���Է�������Ϊ28�����ƶϺ��������Ը÷�Ӧ�ķ���ʽΪ��3O2+C2H4�T2CO2+2H2O��
���ʻ�ѧʽΪC2H4�������ʵ���Է�������Ϊ28�����ƶϺ��������Ը÷�Ӧ�ķ���ʽΪ��3O2+C2H4�T2CO2+2H2O��
�ʴ�Ϊ����1���ټס�������1��8�����û���Ӧ����2����68����3��C2H4��
��������1������ˮú������ȡͼʾ��д�ù��̵ķ�Ӧ����ʽ���ݴ˷���������⼴�ɣ�
��2����2���ٸ��������غ㶨�ɷ�Ӧǰ��ԭ�ӵ����༰��Ŀ���������
�ڸ��ݷ�Ӧ����ʾ��ͼ�����ı仯������ѧ�仯��ʵ�ʣ�
��3���ٸ��������غ㶨�ɣ���������������������ȷ����Ӧ�����������������Ӷ��ó�X��ֵ��
�ڸ���������Ԫ�ص�������ȷ���������ʵ�ԭ�Ӹ����ȣ��Ӷ��õ����ʵĻ�ѧʽ�����ݻ�ѧ����ʽ����д�����д���÷�Ӧ�Ļ�ѧ����ʽ
�������������й������غ㶨�ɵĿ��飬����Ĺؼ�������ö��ɵĺ�ۼ��ۺ��壬��һ�������Ϊȫ����й������غ㶨�ɵĺ��⣮
 CO+H2��
CO+H2�����������Ǻ�������Ԫ��������һ������Ԫ�صĴ�����ʼס��ҡ���������������������Ǽͱ���
�ڼĻ�ѧʽΪH2O�����������Ԫ�ص���������1��2��16=1��8��
�۸÷�Ӧ�ķ�Ӧ�����������һ�ֵ��ʺ�һ�ֻ�����ʸ÷�Ӧ���û���Ӧ��
��2��������Ŀ��������֪�÷�Ӧ��������CD������֮����88g+36g=124g����96g����B�Ƿ�Ӧ����������غ㶨�ɿ�֪�μӷ�Ӧ��B��������124g-96g=28g����������ʣ���B��������96g-28g=68g��
�ڸ��ݸ÷�Ӧ�ķ���ʽ�����֪��Ӧ��Bһ������̼������Ԫ�أ����ܺ�����Ԫ�أ�B����Է�������Ϊ28�����Կ�֪��������ֻ����̼������Ԫ�أ���������ʵĻ�ѧʽΪCxHy�����ڷ�Ӧǰ�����ԭ�ӵĸ�������ȣ���������ǰ�Ļ�ѧ������Ϊ3�����ڷ�Ӧ���������е�̼������Ԫ�ؾ�������CxHy�����Ը�������̼Ԫ�ص�����Ϊ88g��
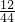 =24g����Ԫ�ص�����Ϊ36g��
=24g����Ԫ�ص�����Ϊ36g��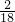 =4g������
=4g������CxHy��̼��Ԫ�ص�����֮��Ϊ
 ��
�� ���ʻ�ѧʽΪC2H4�������ʵ���Է�������Ϊ28�����ƶϺ��������Ը÷�Ӧ�ķ���ʽΪ��3O2+C2H4�T2CO2+2H2O��
���ʻ�ѧʽΪC2H4�������ʵ���Է�������Ϊ28�����ƶϺ��������Ը÷�Ӧ�ķ���ʽΪ��3O2+C2H4�T2CO2+2H2O���ʴ�Ϊ����1���ټס�������1��8�����û���Ӧ����2����68����3��C2H4��
��������1������ˮú������ȡͼʾ��д�ù��̵ķ�Ӧ����ʽ���ݴ˷���������⼴�ɣ�
��2����2���ٸ��������غ㶨�ɷ�Ӧǰ��ԭ�ӵ����༰��Ŀ���������
�ڸ��ݷ�Ӧ����ʾ��ͼ�����ı仯������ѧ�仯��ʵ�ʣ�
��3���ٸ��������غ㶨�ɣ���������������������ȷ����Ӧ�����������������Ӷ��ó�X��ֵ��
�ڸ���������Ԫ�ص�������ȷ���������ʵ�ԭ�Ӹ����ȣ��Ӷ��õ����ʵĻ�ѧʽ�����ݻ�ѧ����ʽ����д�����д���÷�Ӧ�Ļ�ѧ����ʽ
�������������й������غ㶨�ɵĿ��飬����Ĺؼ�������ö��ɵĺ�ۼ��ۺ��壬��һ�������Ϊȫ����й������غ㶨�ɵĺ��⣮

��ϰ��ϵ�д�
�����Ŀ
 ����ʾ��ԭ�ӣ���
����ʾ��ԭ�ӣ��� ����ʾ�������ӣ���ͼ1ΪA��B�������ʷ�Ӧ���۹��̣�ǡ����ȫ��Ӧ�����������ͼʾ�ش��������⣺
����ʾ�������ӣ���ͼ1ΪA��B�������ʷ�Ӧ���۹��̣�ǡ����ȫ��Ӧ�����������ͼʾ�ش��������⣺