��Ŀ����
���ʵ����ʺܴ�̶��Ͼ��������ʵ���;��ijͬѧ����������������̼���п�ȼ�ԣ���˿�����ұ������������ʯ����ˮ�ܷų���������˳����ڸ��������ʳ�������ԣ���˿����ڼ����ɻ����������������ʵ�ɬζ���ܼ�ȩ��ʹ�����ʱ��ԣ���˿�����ʳ��ˮ��Ʒ��������С�մ������ᷴӦ����˿���������θ������ҩ�����������ʵ�������Ӧ�ö�Ӧ��ϵ��ȷ�ĸ�����
A. 1�� B. 2�� C. 3�� D. 4��
 ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д� ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д����������쵪�ʣ����ء�̼淋ȣ������Ϸ��ϡ����ᡢ����ȣ��㷺Ӧ���ڻ������Ṥ��������ҩ���ϳ���ά������
��ҵ�ư����Թ�����ͨ�������������ڸ��¸�ѹ�ʹ����������»������ɵģ����������������ǡ����ȫ��Ӧʱ��������________��
��Ŀǰ��һ�֡��˹��̵������·�����Ӧ����ʾ��ͼ���£�

��1�����ݷ�Ӧ����ʾ��ͼд����ѧ����ʽ��_____________��
��2�������������������ˮ���ã������γɡ����ꡱ��д�������м�Ļ�ѧʽ����������е�Ԫ�صĻ��ϼۣ�_________��
���ǵ��ʵ���Ҫԭ�ϡ�ij���ʳ������̬����(NH4)2SO4�Ĺ����������£�
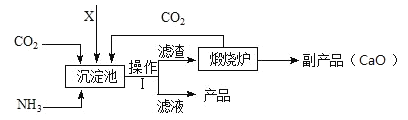
��1���������еIJ�����������_________��
��2�������XӦΪ_____������ĸ��
A H2SO4 B CaSO4 C SO2
������Ҳ�ǹ�ҵ��ά�����ƴ������Ҫԭ�ϡ�

ij��ȤС���������װ��ģ����ά�����Ʊ�̼�����ƣ�ʵ��������£�
a���ر�K1����K2ͨ��NH3�������������ʣ������ȶ���K1ͨ��CO2��
b����������ƿ�ڳ��ֽ϶�NaHCO3����ʱ���ر�K2ֹͣͨNH3��һ��ʱ��ر�K1ֹͣͨCO2��
c����������ƿ�ڵķ�Ӧ�������ˡ�ϴ�ӡ����¸���������ù������ڳ��������м��ȣ���¼ʣ�����������
����ʱ��/min | t0 | t1 | t2 | t3 | t4 | t5 |
ʣ���������/g | δ��¼ | 30.6 | 27.4 | 23.8 | 21.2 | 21.2 |
�����ϣ����³�ѹ�£�1���ˮԼ���ܽ�700���������1���ˮԼ���ܽ�1���������̼��
��ش��������⣺
��1��������ƿ�����ɵ���һ������һ���̬���ʣ�д�����з����Ļ�ѧ��Ӧ����ʽ��__________�����ȹ��˵õ���NaHCO3��������Ӧ��2NaHCO3  Na2CO3 + CO2��+ H2O��
Na2CO3 + CO2��+ H2O��
��2��������ƿ�����ӵij���©������Ҫ������__________��
��3����K2ͨ��NH3һ��ʱ��Ŵ�K1ͨ��CO2��ԭ����__________��
��4������̼��������Һ��������______________��
��5������ʵ���¼������t2 minʱNaHCO3����ķֽ��ʣ��ѷֽ��NaHCO3���������ǰԭNaHCO3�����ı�ֵ������д���������__________��


