��Ŀ����
�����ҹ����ϵ��������������飬���������ˮ���ѣ���ʳ������ˮ��һ�ּ�����Ҫ����Ȼ��Դ��û��ˮ��û�����������ĵ�����1����ѧ���ڻ�������ˮ�������ǣ�______��
��2��С�����ݺ�տ�����һ�����ѧ�˻�ѧ����֪����������Ӳˮ��Խ����к������з����У��ܼ���ˮ�Ƿ�ΪӲˮ����______���ܽ�Ӳˮ�����Ĵ�ʩ��______
A�����ˣ�B����У�C��������D���ӷ���ˮ
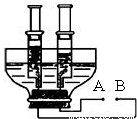
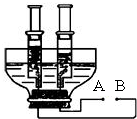
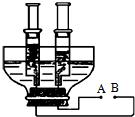
��3����ͼ��С��ͬѧ�ͻ�����Լ�����������ͨ��ֽ�ˮװ�ã��ô�����ƿ�ӽ�ȥƿ�ף���ƿ��һ��Լ8 cm��10 cm��ƿ����һ���������������������öƸ���������ֱ���뽺�������ɵ缫���Ƹ���������Ϸ��ֱ�����ֻ�ݻ���ͬ���ܷ��ԽϺõ���Ͳ�ռ����壮������¶ͷ�����ӵ��ߣ��Իش�
����ͼ��֪B��Ϊ��Դ______������������
������10%������������Һͨ���Բⶨˮ����ɣ�������С�����Ǹ���ʵ����õ����ݣ�����Ϊ������ӽ�����ֵ��һ���ǣ�______��
| ����������g�� | ����������g�� | �������Ƶ��������� | |
| A | 64 | 8 | 9% |
| B | 16 | 2 | 10% |
| C | 16 | 2 | 11% |
| D | 16 | 32 | 11% |
���𰸡���������1��������������벻��ˮ�����ڻ��������ҵ�ˮ�����п����������Ĵ��ڣ�
��2���üӷ���ˮ�ķ����ɼ���Ӳˮ����ˮ������л�����ɽ�Ӳˮ������
��3���ٷּ�ͨ��ֽ�ˮʵ�����������������ɷ������������ڸ���ͨ��ֽ�ˮ�õ������������������Ⱥ�����������Һ�����ʡ��ܼ��������仯���������ѡ����ȷ���ݣ�
����⣺��1��������������벻��ˮ�������ڻ����������ҵ�ˮ�����п����������Ĵ��ڣ�
��2������Ӳˮ����ˮ����ķ�����ʹ�÷���ˮ���飻�����н�Ӳˮ�����ij��÷�������У�
��3���ٸ���ͨ��ֽ�ˮ��ʵ�������֪�����Դ�����������Թ��ڲ������������С�������������Դ�����������Թ��ڲ������������������������B��Ϊ������A��Ϊ������
������10%������������Һͨ���Բⶨˮ����ɣ�����������������������������Ϊ8��1��ͬʱ��Ϊ�������Ƶ��������䣬��ˮ�ֽ��������С�������������Ƶ���������Ӧ����C��������ȷ��
�ʴ�Ϊ����1����ˮ�Ϳ�������������2��D��B����3��������C
�����������ۺϿ���ͨ��ֽ�ˮʵ������ʵ��װ�ã���Ӳˮ��ˮ�ļ����֪ʶ������ʵ�����������ʵ�����������ϵ�������п��飬Ҫ���������ڶԸ�����֪ʶ�������ϼ���������ã�
��2���üӷ���ˮ�ķ����ɼ���Ӳˮ����ˮ������л�����ɽ�Ӳˮ������
��3���ٷּ�ͨ��ֽ�ˮʵ�����������������ɷ������������ڸ���ͨ��ֽ�ˮ�õ������������������Ⱥ�����������Һ�����ʡ��ܼ��������仯���������ѡ����ȷ���ݣ�
����⣺��1��������������벻��ˮ�������ڻ����������ҵ�ˮ�����п����������Ĵ��ڣ�
��2������Ӳˮ����ˮ����ķ�����ʹ�÷���ˮ���飻�����н�Ӳˮ�����ij��÷�������У�
��3���ٸ���ͨ��ֽ�ˮ��ʵ�������֪�����Դ�����������Թ��ڲ������������С�������������Դ�����������Թ��ڲ������������������������B��Ϊ������A��Ϊ������
������10%������������Һͨ���Բⶨˮ����ɣ�����������������������������Ϊ8��1��ͬʱ��Ϊ�������Ƶ��������䣬��ˮ�ֽ��������С�������������Ƶ���������Ӧ����C��������ȷ��
�ʴ�Ϊ����1����ˮ�Ϳ�������������2��D��B����3��������C
�����������ۺϿ���ͨ��ֽ�ˮʵ������ʵ��װ�ã���Ӳˮ��ˮ�ļ����֪ʶ������ʵ�����������ʵ�����������ϵ�������п��飬Ҫ���������ڶԸ�����֪ʶ�������ϼ���������ã�

��ϰ��ϵ�д�
 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�
�����Ŀ
 �����ҹ����ϵ��������������飬���������ˮ���ѣ���ʳ������ˮ��һ�ּ�����Ҫ����Ȼ��Դ��û��ˮ��û�����������ĵ���
�����ҹ����ϵ��������������飬���������ˮ���ѣ���ʳ������ˮ��һ�ּ�����Ҫ����Ȼ��Դ��û��ˮ��û�����������ĵ���
��1����ѧ���ڻ�������ˮ�������ǣ�______��
��2��С�����ݺ�տ�����һ�����ѧ�˻�ѧ����֪����������Ӳˮ��Խ����к������з����У��ܼ���ˮ�Ƿ�ΪӲˮ����______���ܽ�Ӳˮ�����Ĵ�ʩ��______
A�����ˣ�B����У�C��������D���ӷ���ˮ
��3����ͼ��С��ͬѧ�ͻ�����Լ�����������ͨ��ֽ�ˮװ�ã��ô�����ƿ�ӽ�ȥƿ�ף���ƿ��һ��Լ8 cm��10 cm��ƿ����һ���������������������öƸ���������ֱ���뽺�������ɵ缫���Ƹ���������Ϸ��ֱ�����ֻ�ݻ���ͬ���ܷ��ԽϺõ���Ͳ�ռ����壮������¶ͷ�����ӵ��ߣ��Իش�
����ͼ��֪B��Ϊ��Դ______������������
������10%������������Һͨ���Բⶨˮ����ɣ�������С�����Ǹ���ʵ����õ����ݣ�����Ϊ������ӽ�����ֵ��һ���ǣ�______��
| ����������g�� | ����������g�� | �������Ƶ��������� | |
| A | 64 | 8 | 9% |
| B | 16 | 2 | 10% |
| C | 16 | 2 | 11% |
| D | 16 | 32 | 11% |
 15�������ҹ����ϵ��������������飬���������ˮ���ѣ���ʳ������ˮ��һ�ּ�����Ҫ����Ȼ��Դ��û��ˮ��û�����������ĵ���
15�������ҹ����ϵ��������������飬���������ˮ���ѣ���ʳ������ˮ��һ�ּ�����Ҫ����Ȼ��Դ��û��ˮ��û�����������ĵ���
 ������Դ��������������Ļ���������4��22�ա���������ա��������ǡ���ϧ������Դ��ת�䷢չ��ʽ����
������Դ��������������Ļ���������4��22�ա���������ա��������ǡ���ϧ������Դ��ת�䷢չ��ʽ����