��Ŀ����
ʵ�������÷�Ӧ��������ͭ��ϡ������Һ�Ʊ�CuSO4��5H2O������Ҫ������ͼ��

��֪:20 ��ʱ������ͭ���ܽ��Ϊ20.7g ��
��1���Լ�XӦѡ��__������ţ���
a Cu b CuO c Fe
��2������AΪ______�����������ò���������___�����������ձ���
��3������BΪ_____�����µ�20��ᾧ�����ˡ����˺���Һ��������������Ϊ___�� �������ȷ��0.1%��
��4������CΪ��ˮϴ�ӡ����¸���ñ�ˮϴ�ӵ�Ŀ��;
����_________������__________��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ




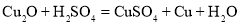 ��
�� �����
����ë�� �ѺϽ����
�ѺϽ���� ����Ͱ
����Ͱ �ѺϽ��
�ѺϽ��