��Ŀ����
��2010?������2009��12���ڸ籾�����ٿ������Ϲ�����仯��ᣬ����̼�����Ϊ�ȵ㻰�⣮����̼�����ָ������Ϣʱ����������Ҫ�������٣��Ӷ�����̼�ر��Ƕ�����̼���ŷţ���1�������������ж�����̼����������������Ҫԭ����______����ɵ�Σ����______������һ�㣩��
��2����Ȼ�������Ķ�����̼����Ҫ;����______����ѧ�������о�������Ķ�����̼��������һ�������·�Ӧת���ɼ����ˮ���÷�Ӧ�Ļ�ѧ����ʽ��______.CH4+2H2O
���𰸡���������1�����ݽ�������ҵ��չ״����ɭ�ֵ��ƻ�����ش�ǰһ�գ����ݶ�����̼�Ĵ����ŷŶԻ�����Ӱ��ش��һ�գ�
��2��������Ȼ���е�̼��ѭ���ش�ǰһ�գ�������Ϣ��������Ķ�����̼��������һ�������·�Ӧת���ɼ����ˮ���ش��һ�գ�
��3�������ճ������н��ܼ��ŵ�ʵ���ش�
����⣺��1�������������ִ���ҵ�ķ�չ�������˴����Ļ�ʯȼ�ϣ�����ȼ�ղ����˴����Ķ�����̼��ͬʱ����ɭ�ֵ������ƻ���ͨ������������յĶ�����̼���٣����Դ����ж�����̼����������������Ҫԭ���ǻ�ʯȼ�ϵ�ȼ�պ�ɭ����ƻ���
��2�����������ֲ��ͨ��Ҷ���壬���ù��ܣ��Ѷ�����̼��ˮ�ϳɵ��ۺ�����ͬʱ�ͷ������Ĺ��̣�����Ȼ�������Ķ�����̼����Ҫ;����������Ϣ��������Ķ�����̼��������һ�������·�Ӧת���ɼ����ˮ�����÷�Ӧ�Ļ�ѧ����ʽ��CO2+4H2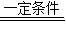 CH4+2H2O��
CH4+2H2O��
��3���ճ������н��ܼ��ŵ�ʵ���ܶ࣬�粻ʹ��һ���Կ��ӣ����Բ��д���˳�������������Ȼ�����ƹ�ȣ�
�ʴ�Ϊ����1����ʯȼ�ϵ�ȼ�գ���ɭ����ƻ��Ⱥ����𰸾��ɣ�������ЧӦ����ȫ�������ů�����������ڻ�������ɳĮ���Ⱥ����𰸾��ɣ�
��2��ֲ��Ĺ�����ã��������ã�
CO2+4H2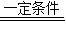 CH4+2H2O����3����ʹ��һ���Կ��ӣ����Բ��д���˳�����������Ȼ�����ƹ����Ͻ��ܼ��ŵĺ����𰸾��ɣ���
CH4+2H2O����3����ʹ��һ���Կ��ӣ����Բ��д���˳�����������Ȼ�����ƹ����Ͻ��ܼ��ŵĺ����𰸾��ɣ���
������������Ҫ�����������������̼��ͬѧ���˽��˶�����̼�����������Ҫԭ���Լ���μ��ٶ�����̼���壬�ͻ����ô��⣮
��2��������Ȼ���е�̼��ѭ���ش�ǰһ�գ�������Ϣ��������Ķ�����̼��������һ�������·�Ӧת���ɼ����ˮ���ش��һ�գ�
��3�������ճ������н��ܼ��ŵ�ʵ���ش�
����⣺��1�������������ִ���ҵ�ķ�չ�������˴����Ļ�ʯȼ�ϣ�����ȼ�ղ����˴����Ķ�����̼��ͬʱ����ɭ�ֵ������ƻ���ͨ������������յĶ�����̼���٣����Դ����ж�����̼����������������Ҫԭ���ǻ�ʯȼ�ϵ�ȼ�պ�ɭ����ƻ���
��2�����������ֲ��ͨ��Ҷ���壬���ù��ܣ��Ѷ�����̼��ˮ�ϳɵ��ۺ�����ͬʱ�ͷ������Ĺ��̣�����Ȼ�������Ķ�����̼����Ҫ;����������Ϣ��������Ķ�����̼��������һ�������·�Ӧת���ɼ����ˮ�����÷�Ӧ�Ļ�ѧ����ʽ��CO2+4H2
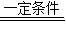 CH4+2H2O��
CH4+2H2O����3���ճ������н��ܼ��ŵ�ʵ���ܶ࣬�粻ʹ��һ���Կ��ӣ����Բ��д���˳�������������Ȼ�����ƹ�ȣ�
�ʴ�Ϊ����1����ʯȼ�ϵ�ȼ�գ���ɭ����ƻ��Ⱥ����𰸾��ɣ�������ЧӦ����ȫ�������ů�����������ڻ�������ɳĮ���Ⱥ����𰸾��ɣ�
��2��ֲ��Ĺ�����ã��������ã�
CO2+4H2
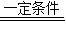 CH4+2H2O����3����ʹ��һ���Կ��ӣ����Բ��д���˳�����������Ȼ�����ƹ����Ͻ��ܼ��ŵĺ����𰸾��ɣ���
CH4+2H2O����3����ʹ��һ���Կ��ӣ����Բ��д���˳�����������Ȼ�����ƹ����Ͻ��ܼ��ŵĺ����𰸾��ɣ���������������Ҫ�����������������̼��ͬѧ���˽��˶�����̼�����������Ҫԭ���Լ���μ��ٶ�����̼���壬�ͻ����ô��⣮

��ϰ��ϵ�д�
�����Ŀ