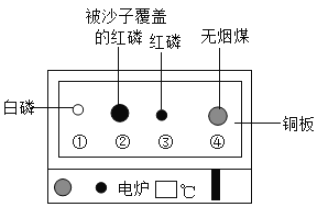
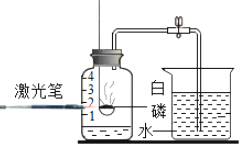
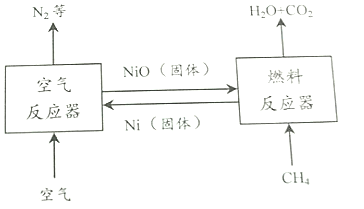
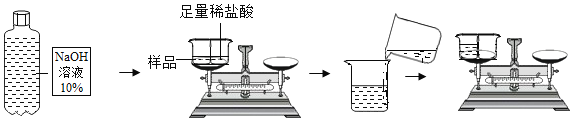
��Ŀ����
����Ŀ��(1)ij�������ķ�Һ����Ҫ����������������ͭ��ȡһ�����ĸ÷�Һ��Ʒ������þ�ۺ�ͭ�ۣ���ַ�Ӧ����ˣ��õ���Һ������������������ͭ�ۣ�����Һ�е�����һ������____��(��д��ѧʽ����ͬ)���ܺ���____����Ӧ��õ�����Һ������____(����С����������������������)��Ӧǰ��ȡ��Һ��Ʒ��������
(2)����Al�����ܺ����ᷴӦ�����ܺ��������Ʒ�Ӧ����֪Al������������Һ��Ӧ����NaAlO2��H2������д��Al������������Һ��Ӧ�Ļ�ѧ��Ӧ����ʽ_____
(3)�ó�ϴ��δ���ɵIJ�����պȡ����Һ�ⶨpHֵ�ᵼ�²�ý��_____(����ƫ������ƫС����������������һ����)��
���𰸡�Mg(NO3)2 Cu(NO3)2 С�� ![]() ��һ��
��һ��
��������
��1����������ǰ��Ľ������Խ����ں���Ľ�����������Һ���û���������þ��ͭ���ã�ͭ�������ã�������ͭ�ۺ�þ�۵Ļ�������һ��������������������ͭ��Һ�У�þ�Ⱥ���������Ӧ�����������������þ��ȫ��Ӧ��ͭ�ٺ���������Ӧ��
��2�����ݷ�Ӧ�����������д��ѧ����ʽ��
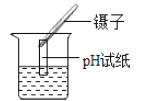
��3��������pH��ֽ�ⶨδ֪��Һ��pH�ķ������з���
��1������������ͭ�ۣ�˵��������һ������Ӧ�꣬þ�Ƿ��������ȷ������þһ���μӷ�Ӧ��������þ������ͭ�Ƿ�Ӧ����ȷ���������Һ��һ����������þ�����ܺ�������ͭ��һ��û�������������Mg��NO3��2��Cu��NO3��2��
���ܷ�����Ӧ�Ļ�ѧ����ʽΪ��
![]()
![]()
![]()
�ɷ�Ӧ��������ϵ��֪����Ӧ��õ��������������ڷ�Ӧǰ����þ�ۡ�ͭ�۵���������˷�Ӧ��õ���Һ������С�ڷ�Ӧǰ��ȡ��Һ��Ʒ�����������С�ڡ�
��2��Al������������Һ��Ӧ����NaAlO2��H2�Ļ�ѧ����ʽΪ��![]() ��
��
��3���ⶨ��ҺpHʱ��ϴ����δ���ɵIJ�����պȡ����Һ��ϡ���˴�����Һ�������������Һ��ʹ��Һ�����Լ��������²�ý��ƫ������������Һ��ʹ��Һ�ļ��Լ��������²�ý��ƫС�������������Һ����ý�����䡣�����һ����