��Ŀ����
�ҹ�Ŀǰʹ�õ�ȼ����Ҫ��ú��ʯ�ͣ��������úȼ��ʱ���ɵ�SO2����Ⱦ��������1��ij�������糧ÿ��ȼ�պ���1.6%��ú100t����ú�е���ȫ��ת��ΪSO2����ó�ÿ�����SO2
��2�����ұ��涨��ҵ������SO2�������ó���0.15mg/m3����ҵ�ϲ���SO2�ĺ���ʱ�����Ը��ݷ�Ӧ��SO2+2H2O+I2=H2SO4+2HI����ȡ�ó�������Ʒ1000L����0.0254%�ĵ⣨12����Һ2g����ȫ��Ӧ���Լ���ó��ŷŵķ�����SO2�ĺ����Ƿ���Ϲ��ұ���
���������ݷ�Ӧ��������P�������غ㶨�ɿ�����ȷ����д��ѧ����ʽ��������Ԫ�ص������Ͷ�����������Ԫ�ص��������������Լ���ó�������������������ݷ�Ӧ�Ļ�ѧ����ʽ��������ó��ŷŵķ�����SO2�ĺ����Ƿ���Ϲ��ұ���
����⣺��1��ÿ��ʹ��ú���е��������Ϊ100t��1.6%�����ڶ�����������Ԫ�ص���������Ϊ
��100%=50%�����Ըó�ÿ������������������Ϊ��100t��1.6%��50%=3.2t�����3.2��
����������������Ʒ�Ӧ��������������ƣ���Ӧ�Ļ�ѧ����ʽΪ��2SO2+Ca��OH��2�TCa��HSO3��2
��2����������������ΪX��
SO2+2H2O+I2=H2SO4+2HI
64 254
X 2g��0.0254%
=
X=1.28��10-4g
��l000L������SO2������Ϊ1.28��10-4g��
1.28��10-4g=O.128mg/m3��0.15mg/m3�����Ϲ��ұ���
| 32��1 |
| 32��1+16��2 |
����������������Ʒ�Ӧ��������������ƣ���Ӧ�Ļ�ѧ����ʽΪ��2SO2+Ca��OH��2�TCa��HSO3��2
��2����������������ΪX��
SO2+2H2O+I2=H2SO4+2HI
64 254
X 2g��0.0254%
| 64 |
| 254 |
| X |
| 2g��0.0254% |
X=1.28��10-4g
��l000L������SO2������Ϊ1.28��10-4g��
1.28��10-4g=O.128mg/m3��0.15mg/m3�����Ϲ��ұ���
�����������Ҫ����������ط���ļ��㷽����ֻ����������ط���ļ��㷽������ͨ������ó���ȷ�Ĵ𰸣�

��ϰ��ϵ�д�
 һ����������ϵ�д�
һ����������ϵ�д�
�����Ŀ
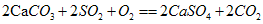 ��ʵ��֤��ʯ�ҽ�[Ca(OH)2]�ڿ�����Ҳ������SO2��������ƺ��������ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ��________________________________��
��ʵ��֤��ʯ�ҽ�[Ca(OH)2]�ڿ�����Ҳ������SO2��������ƺ��������ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ��________________________________��