��Ŀ����
��2002?�Ƹԣ�Ϊ�˲ⶨijʯ��ʯ�����Ĵ��ȣ������������ʲ����ᷴӦ����ijͬѧ���������̽���ʵ�飺�������ܽ���������������������NaOH��Һ�������������NaOH��Һ����������������Ĵ��ȣ�ʵ���������ȡ����������Ϊ10g��ʵ��װ������ͼ��ʾ��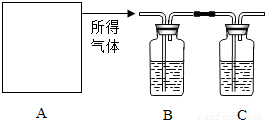
��1��AΪ�ܽ������װ�ã����Ѿ����˳���©����˫�������������ܣ�Ϊ������ܽ����ٻ���Ҫ��ʵ��������______
��2��A�з�����Ӧ��ѧ����ʽ______
��3��B����ʢ���Լ���______
��4����������װ�ý���ʵ�飬���������ʹʵ��������ƫ�����______
��ʵ�鿪ʼA��Bװ���Լ��������ڲ�������������
�ڽ���Cװ�õ������л���������HCl����
������ͨ���ٶȹ��죬CO2��������NaOH��Һȫ������
��ʵ�����ʱ��ϵͳ�ڻ�����������CO2����
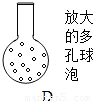
��5����������Һ�Ĺ��ӵ��¶˸ijɾ��ж�����ݣ�ͼ�е�D�������������ʵ���ȷ�ȣ���������______
��6���Ľ�ʵ��װ�ò�������ȷ��������ȷ��������Ĵ��ȣ�����ʱCװ����ʵ��ǰ��������������3.6 g����ÿ����Ĵ���Ϊ______��
���𰸡���������1����֪Ϊ������ܽ����ٻ���Ҫ��ʵ��������ʲô����֪��ʵ�鹲����Щ������
��2����дA�з�����Ӧ��ѧ����ʽ����֪ʯ��ʯ������Ļ�ѧʽ������֪�䷴Ӧ�����
��3��Ũ���������ˮ�ԣ������������
��4������ȷѡ�����μ�ʵ����ע������
��5����������Һ�Ĺ��ӵ��¶˸ijɾ��ж�����ݺ����������������Һ�ĽӴ������ʹ���屻������գ�
��6��Cƿ�ж�����������������ɵ�CO2�����������ݷ���ʽCaCO3+2HCl=CaCl2+H2O+CO2���ɼ����CaCO3������������̼��Ƶ���������= �ɼ����������������
�ɼ����������������
����⣺��1��Ҫ��ɴ�ʵ�飬��Ҫ��ʵ�������г���©����˫�������������ܡ����ƿ������ƿ�����Թܡ���ƿ�����ʴ�Ϊ���ƿ������ƿ�����Թܡ���ƿ����
��2��ʯ��ʯ�Ļ�ѧʽ��CaCO3������Ļ�ѧʽΪHCl����A�з�����Ӧ�ķ���ʽΪCaCO3+2HCl=CaCl2+H2O+CO2����
��3��Ũ���������ˮ�ԣ��������������B����ʢ���Լ���Ũ���
��4����ʵ�鿪ʼA��Bװ���Լ��������ڲ���������������������Һʹʵ�鲻��ȷ��������ȷ���㷴Ӧ������ɣ�
��˵����ǰ��ķ�Ӧ������û����ȫ��Ӧ��
������ͨ���ٶȹ��죬CO2��������NaOH��Һȫ�����գ���ʹ��������ȷ��
��ʵ�����ʱ��ϵͳ�ڻ�����������CO2���壬˵��CO2û�б�NaOH��Һȫ�����գ���ʹ��������ȷ��
��5��������ݿ���������������Һ�ĽӴ������ʹ���屻������գ����������ʵ���ȷ�ȣ��ʴ�Ϊ������������������Һ�ĽӴ������ʹ���屻������գ�
��6���⣺����Ʒ��CaCO3������Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 3.6g
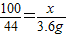
���x��8.2g
��Ʒ��CaCO3����������Ϊ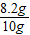 ×100%=82%
×100%=82%
�𣺸ÿ����Ĵ���Ϊ82%��
������������Ҫ����ѧ����ʵ����������պ����ʴ��ȵļ��㣬ѧ�������μDz���ʵ�鲽���ע���������������������������ʽ���м��㣬������ȷ���
��2����дA�з�����Ӧ��ѧ����ʽ����֪ʯ��ʯ������Ļ�ѧʽ������֪�䷴Ӧ�����
��3��Ũ���������ˮ�ԣ������������
��4������ȷѡ�����μ�ʵ����ע������
��5����������Һ�Ĺ��ӵ��¶˸ijɾ��ж�����ݺ����������������Һ�ĽӴ������ʹ���屻������գ�
��6��Cƿ�ж�����������������ɵ�CO2�����������ݷ���ʽCaCO3+2HCl=CaCl2+H2O+CO2���ɼ����CaCO3������������̼��Ƶ���������=
 �ɼ����������������
�ɼ��������������������⣺��1��Ҫ��ɴ�ʵ�飬��Ҫ��ʵ�������г���©����˫�������������ܡ����ƿ������ƿ�����Թܡ���ƿ�����ʴ�Ϊ���ƿ������ƿ�����Թܡ���ƿ����
��2��ʯ��ʯ�Ļ�ѧʽ��CaCO3������Ļ�ѧʽΪHCl����A�з�����Ӧ�ķ���ʽΪCaCO3+2HCl=CaCl2+H2O+CO2����
��3��Ũ���������ˮ�ԣ��������������B����ʢ���Լ���Ũ���
��4����ʵ�鿪ʼA��Bװ���Լ��������ڲ���������������������Һʹʵ�鲻��ȷ��������ȷ���㷴Ӧ������ɣ�
��˵����ǰ��ķ�Ӧ������û����ȫ��Ӧ��
������ͨ���ٶȹ��죬CO2��������NaOH��Һȫ�����գ���ʹ��������ȷ��
��ʵ�����ʱ��ϵͳ�ڻ�����������CO2���壬˵��CO2û�б�NaOH��Һȫ�����գ���ʹ��������ȷ��
��5��������ݿ���������������Һ�ĽӴ������ʹ���屻������գ����������ʵ���ȷ�ȣ��ʴ�Ϊ������������������Һ�ĽӴ������ʹ���屻������գ�
��6���⣺����Ʒ��CaCO3������Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 3.6g
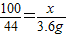
���x��8.2g
��Ʒ��CaCO3����������Ϊ
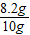 ×100%=82%
×100%=82%�𣺸ÿ����Ĵ���Ϊ82%��
������������Ҫ����ѧ����ʵ����������պ����ʴ��ȵļ��㣬ѧ�������μDz���ʵ�鲽���ע���������������������������ʽ���м��㣬������ȷ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
��2002?�Ƹԣ�Ϊ�ⶨijͭп�Ͻ���п������������ijͬѧ���úϽ���ϡ���ᷴӦ������������ʵ�飬������ص�ʵ�����ݼ�¼���£�ʵ���е������Բ��ƣ�
��1���Լ���ͭп�Ͻ���п����������______��
��2�����ϱ����ݷ���������ȡ�Ͻ�������ϡ�����������______ʱ�������Ͻ��е�п��ϡ�����е�����ǡ����ȫ��Ӧ����ʱϡ�������������Ϊ______��
| ��һ�� | �ڶ��� | ������ | |
| ��ȡ�Ͻ������/g | 25 | 25 | 50 |
| ����ϡ���������/g | 120 | 160 | 100 |
| ��������������/g | 0.4 | 0.4 | 0.4 |
��2�����ϱ����ݷ���������ȡ�Ͻ�������ϡ�����������______ʱ�������Ͻ��е�п��ϡ�����е�����ǡ����ȫ��Ӧ����ʱϡ�������������Ϊ______��
��2002?�Ƹԣ�Ϊ�ⶨijͭп�Ͻ���п������������ijͬѧ���úϽ���ϡ���ᷴӦ������������ʵ�飬������ص�ʵ�����ݼ�¼���£�ʵ���е������Բ��ƣ�
��1���Լ���ͭп�Ͻ���п����������______��
��2�����ϱ����ݷ���������ȡ�Ͻ�������ϡ�����������______ʱ�������Ͻ��е�п��ϡ�����е�����ǡ����ȫ��Ӧ����ʱϡ�������������Ϊ______��
| ��һ�� | �ڶ��� | ������ | |
| ��ȡ�Ͻ������/g | 25 | 25 | 50 |
| ����ϡ���������/g | 120 | 160 | 100 |
| ��������������/g | 0.4 | 0.4 | 0.4 |
��2�����ϱ����ݷ���������ȡ�Ͻ�������ϡ�����������______ʱ�������Ͻ��е�п��ϡ�����е�����ǡ����ȫ��Ӧ����ʱϡ�������������Ϊ______��
 3N2��+5CO2��+19H2O��������Ӧ��B��Ӧ��������ʵĻ�ѧʽ�ǣ�
3N2��+5CO2��+19H2O��������Ӧ��B��Ӧ��������ʵĻ�ѧʽ�ǣ�