��Ŀ����
��8�֣���ѧʵ����������ڻ�ѧѧϰ���о��о�����Ҫ���á�����a �Թܡ�b ©���� c �ƾ��ơ�d����ƿ�� eҩ�ס� f ��ͷ�ιܡ� g��Ͳ(10mL,50mL,100mL)����������Ϊ����ʵ�������ѡһ�֡�
����������Դ�������� �����������գ�
����ȡ��μ�����Һ��ʹ�� �����������գ�
�ǿ���ֱ���ھƾ��ƻ����ϼ��ȵIJ��������� �����������գ�
��ijͬѧ����98����Ũ���ᣨ�ܶ�Ϊ1.84g/cm3��������150g 10����ϡ���ᡣ
����ɱ�ʵ��������ṩ�������⣬����Ҫ�IJ��������� ��
��������Һ�����У���Ҫ��ȡ mL�����������0.1����Ũ���ᣬѡ�� mL����Ͳ������ʱ����ͬѧ������Ͳ�Ŀ̶��ߣ�������������������Ũ�������� ���ƫ��ƫС������Ӱ�족����
����������Դ�������� �����������գ�
����ȡ��μ�����Һ��ʹ�� �����������գ�
�ǿ���ֱ���ھƾ��ƻ����ϼ��ȵIJ��������� �����������գ�
��ijͬѧ����98����Ũ���ᣨ�ܶ�Ϊ1.84g/cm3��������150g 10����ϡ���ᡣ
����ɱ�ʵ��������ṩ�������⣬����Ҫ�IJ��������� ��
��������Һ�����У���Ҫ��ȡ mL�����������0.1����Ũ���ᣬѡ�� mL����Ͳ������ʱ����ͬѧ������Ͳ�Ŀ̶��ߣ�������������������Ũ�������� ���ƫ��ƫС������Ӱ�족����
��1��c ��2��f ��3��a
��4���ٲ��������ձ� ��8.3mL�� 10mL�� ƫС
��4���ٲ��������ձ� ��8.3mL�� 10mL�� ƫС
�����������1�����ݳ�����������;�������ƾ��ƿ�������Դ����ѡc��
��2����ͷ�ι�������ȡ��μ�����Һ�壬��ѡf��
��3����ֱ���ھƾ����ϼ��ȵ��������Թܡ�������������ȼ�ճף�����ֱ���ھƾ����ϼ��ȵIJ�������ֻ���Թܣ���ѡa��
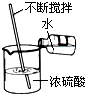
��4���ٸ�������һ������������������Һ�IJ��裨���㡢�������ܽ⣩����ȷ�����õ�����������һ������������������Һ����Ҫeҩ�ס�f��ͷ�ιܡ�g��Ͳ�⣬��Ҫ�õ����������ձ���
������Һϡ�͵�ǰ�ı�����ܼ����������������������䡣
�裺����150g 10%��ϡ���ᣬ��Ҫ98%��Ũ����Xg
��150g��10%=X?98%
���X=15.3g
����m=��v����֪v=
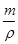 =
=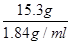 =8.3ml
=8.3ml��������Ͳ��ȡһ���������Һ��ʱ��Ӧ����ѡȡһ����ȡȫ��Һ�����С������Ͳ����ѡ��10ml����Ͳ�����ڸ��Ӷ���ʱ��ʵ��ֵƫ��������ȡŨ����������ƫС��
��������ѧ����ʵ��Ϊ������ѧ�ƣ���Ϥ����������ʹ�÷�����ע��������ջ�ѧʵ����������ǽ�������Ŀ�Ĺؼ���

��ϰ��ϵ�д�
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ