��Ŀ����
��ͼ��1994���2004��ij���е�һ�����ʱ�̲�Ŀ����ж�������ĺ���������˵��������ǣ� ��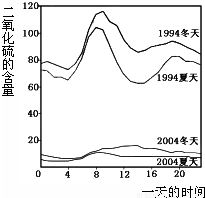
A��������ʾ��������еĶ����������������
B��������ʾ��1994��һ���д�Լ8�����Ҷ�������ĺ����ϸ�
C����������ĺ�����10��併�͵�ԭ������Ǽ�ǿ��ȼ�ϵ�����Ϳ����˶���������ŷ�
D������������Ⱦ����Ҫ��Դ�������ŷŵ�β����������Ⱦ�ķ����ǽ�ֹʹ������
���𰸡�������A���۲�ͼʾ��֪����������еĶ���������������ߣ�
B���۲�1994�궬������������ͼ�жϣ�
C�������еĶ���������Ҫ����ú�Ȼ�ʯȼ�ϵ�ȼ�պ����Ṥ���ŷŵ�β����
D�����ݶ����������Դ�ͷ����Ŀ����Իش�
����⣺A���۲�ͼʾ��֪��������ʾ��������еĶ���������������ߣ���ȷ��
B���۲�ͼʾ��֪��������ʾ��1994��һ���д�Լ8�����Ҷ�������ĺ����ϸߣ���ȷ��
C�����ݿ����еĶ���������Ҫ����ú�Ȼ�ʯȼ�ϵ�ȼ�պ����Ṥ���ŷŵ�β����֪����������ĺ�����10��併�͵�ԭ������Ǽ�ǿ��ȼ�ϵ�����Ϳ����˶���������ŷţ�����ȷ��
D������������Ҫ����ú�Ȼ�ʯȼ�ϵ�ȼ�պ����Ṥ���ŷŵ�β�����ھ��ǽ�ֹʹ�������Dz���ʵ�ʵģ��ʴ���
��ѡD��
������Ҫ���ٴ����ж�������ĺ���������ǿ��ȼ�ϵ�����Ϳ����˶���������ŷ����⣬��Ӧ�������͵Ļ�����Դ���Ӷ������������Ⱦ���⣮
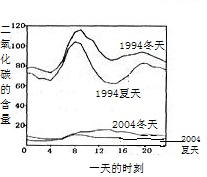
B���۲�1994�궬������������ͼ�жϣ�
C�������еĶ���������Ҫ����ú�Ȼ�ʯȼ�ϵ�ȼ�պ����Ṥ���ŷŵ�β����
D�����ݶ����������Դ�ͷ����Ŀ����Իش�
����⣺A���۲�ͼʾ��֪��������ʾ��������еĶ���������������ߣ���ȷ��
B���۲�ͼʾ��֪��������ʾ��1994��һ���д�Լ8�����Ҷ�������ĺ����ϸߣ���ȷ��
C�����ݿ����еĶ���������Ҫ����ú�Ȼ�ʯȼ�ϵ�ȼ�պ����Ṥ���ŷŵ�β����֪����������ĺ�����10��併�͵�ԭ������Ǽ�ǿ��ȼ�ϵ�����Ϳ����˶���������ŷţ�����ȷ��
D������������Ҫ����ú�Ȼ�ʯȼ�ϵ�ȼ�պ����Ṥ���ŷŵ�β�����ھ��ǽ�ֹʹ�������Dz���ʵ�ʵģ��ʴ���
��ѡD��
������Ҫ���ٴ����ж�������ĺ���������ǿ��ȼ�ϵ�����Ϳ����˶���������ŷ����⣬��Ӧ�������͵Ļ�����Դ���Ӷ������������Ⱦ���⣮

��ϰ��ϵ�д�
 ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
�����Ŀ
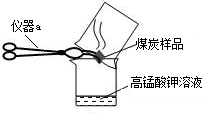
 ����Ȼ����ѧ����ʽ�����һ�����ʵĻ�ѧʽӡˢ������������������˽��������һ���ᣬ�������ѧ֪ʶ�Ʋ��仯ѧʽ��
����Ȼ����ѧ����ʽ�����һ�����ʵĻ�ѧʽӡˢ������������������˽��������һ���ᣬ�������ѧ֪ʶ�Ʋ��仯ѧʽ��