��Ŀ����
����Ŀ��ijͬѧ���á�����ŵ���ơ�ЧӦ���������ͼ������ȡ��������֤�����ʵ�顣��A�з�Һ©���Ļ������ܿ쿴��E�е��ܿ�������ð��������Һ����ɫ���ɫ�����ͼ�ش����⣺
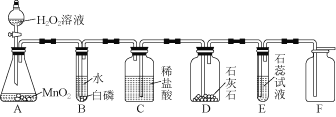
��1��A�з�Ӧ�Ļ�ѧ����ʽ____________________��B�а��ײ�ȼ�գ�ԭ����________��
��2��C��D�п���������ֱ���____________________��____________________������D�з�Ӧ�Ļ�ѧ����ʽ______________��
��3���û�ѧ����ʽ��ʾE����ɫ�仯��ԭ��____________________��
��4����Fװ���ռ������������____________________���÷���ʽ��ʾ��֤Fװ���е�����_________��
���𰸡�2H2O2![]() 2H2O+O2�� �¶�û�дﵽ�����Ż�� C��Һ�屻ѹ��D��/C��Һ���½� ������������ CaCO3 + 2HCl = CaCl2 + H2O + CO2�� H2O + CO2=H2CO3 CO2 �ܶȱȿ����� Ca(OH)2 + CO2 = CaCO3 ��+ H2O
2H2O+O2�� �¶�û�дﵽ�����Ż�� C��Һ�屻ѹ��D��/C��Һ���½� ������������ CaCO3 + 2HCl = CaCl2 + H2O + CO2�� H2O + CO2=H2CO3 CO2 �ܶȱȿ����� Ca(OH)2 + CO2 = CaCO3 ��+ H2O
��������
��1�����������ڶ������̵Ĵ������£��ֽ�����ˮ����������Ӧ�ķ���ʽΪ��2H2O2![]() 2H2O+O2���������������Ӵ�ʱû��ȼ�գ�˵���¶�û�дﵽ�����Ż�㣻
2H2O+O2���������������Ӵ�ʱû��ȼ�գ�˵���¶�û�дﵽ�����Ż�㣻
��2��Cװ���У������������࣬ѹǿ����ϡ����ѹ��D�У��ܹ��۲쵽C��Һ���½���ϡ������ʯ��ʯ�Ӵ���Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���ܹ��۲쵽D��ϡ������룬�д������ݲ�������Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
��3��E�е��ܿ�������ð����˵��������̼����ʯ����Һ�У�������̼�ܺ�ʯ����Һ�е�ˮ��Ӧ����̼�ᣬ��Ӧ�Ļ�ѧ����ʽ��H2O+CO2=H2CO3��̼�������ԣ���ʹʯ����Һ���ɫ�������Һ����ɫ��Ϊ��ɫ��
��4����Ϊ������̼���ܶȱȿ����Ĵ��������������ſ������ռ������������̼��ԭ���Ƕ�����̼��ʹ����ʯ��ˮ���������Ӧ�ķ���ʽΪ��Ca(OH)2+CO2=CaCO3 ��+H2O��

����Ŀ��ij��ȤС���ʯ��ʯ��Ʒ��������ʵ��ȡ12g��Ʒ�����ձ��У���100gϡ�����4�μ��뵽�ձ��У���ַ�Ӧ�����ʲ�����ˮ��Ҳ�����ᷴӦ�������ʣ������������¼���¡�����㣺
���� | 1 | 2 | 3 | 4 |
����ϡ���������/g | 25 | 25 | 25 | 25 |
ʣ����������/g | 8 | a | 2 | 2 |
��
��1��������a=_____
��2����Ʒ��̼��Ƶ�����Ϊ_____g��
��3����4�μ���ϡ�����������Һ��CaCl2������____?��д��������̣���