��Ŀ����
����������мг���������ʡ�ԣ��������ͼ�ش����⣺��12�֣�
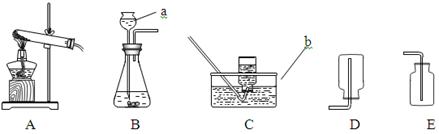
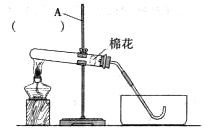
��1��ͼ�������ܢݵ����Ƣ� ���� ��
��2��ʵ������ȡ������ijͬѧ�����ͼA��װ�ã������������ռ����������У���������ʢ�ŵ��Լ�ӦΪ ���˷����������Ļ�ѧ����ʽΪ ��
�������ˮ���ռ��������벹������A�м���ƿ�еĵ��ܡ�
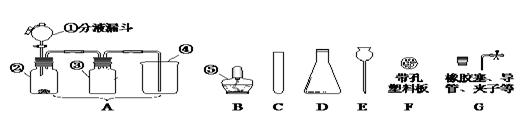
��3��ʵ������ȡ������̼����B~G��ѡ����������װ����װ�ã�Ҫ���ܷ�����Ʒ�Ӧ�ķ�����ֹͣ�������ȷѡ��Ϊ������ĸ�� ����Ӧ�Ļ�ѧ����ʽΪ ��
��4��ʵ����ͨ��������ͼ��ʾ��ϴ��װ�ÿ����ڼ��������̼���壬ϴ��ƿ������װ��ҩƷ�����ǣ� ������ĸ����
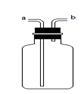
A������ʯ��ˮ B����ɫʯ����Һ C��ˮ
�йط�Ӧ�Ļ�ѧ����ʽΪ ��
��5����ѡ����ͼװ�����ռ�CO2��������Ӧ�� �ˣ��a����b�������롣

��1��ͼ�������ܢݵ����Ƣ� ���� ��
��2��ʵ������ȡ������ijͬѧ�����ͼA��װ�ã������������ռ����������У���������ʢ�ŵ��Լ�ӦΪ ���˷����������Ļ�ѧ����ʽΪ ��
�������ˮ���ռ��������벹������A�м���ƿ�еĵ��ܡ�
��3��ʵ������ȡ������̼����B~G��ѡ����������װ����װ�ã�Ҫ���ܷ�����Ʒ�Ӧ�ķ�����ֹͣ�������ȷѡ��Ϊ������ĸ�� ����Ӧ�Ļ�ѧ����ʽΪ ��
��4��ʵ����ͨ��������ͼ��ʾ��ϴ��װ�ÿ����ڼ��������̼���壬ϴ��ƿ������װ��ҩƷ�����ǣ� ������ĸ����

A������ʯ��ˮ B����ɫʯ����Һ C��ˮ
�йط�Ӧ�Ļ�ѧ����ʽΪ ��
��5����ѡ����ͼװ�����ռ�CO2��������Ӧ�� �ˣ��a����b�������롣
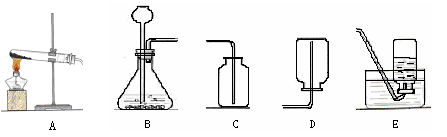
��1���� �ձ� ���ݾƾ��ơ�
��2�� ����������Һ��
��ѧ����ʽΪ 2H2O2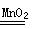 2H20+O2��
2H20+O2��
�벹��������ͼ��
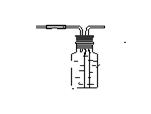
��3��������ĸ�� CEFG����ѧ����ʽΪCaCO3+2HCl===CaCl2+CO2 + H2O ��
��4�� A ������ĸ������ѧ����ʽΪ CO2+ Ca(OH)2====CaCO3+ H2O ��
��5�� a���롣
��2�� ����������Һ��
��ѧ����ʽΪ 2H2O2
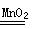 2H20+O2��
2H20+O2���벹��������ͼ��

��3��������ĸ�� CEFG����ѧ����ʽΪCaCO3+2HCl===CaCl2+CO2 + H2O ��
��4�� A ������ĸ������ѧ����ʽΪ CO2+ Ca(OH)2====CaCO3+ H2O ��
��5�� a���롣
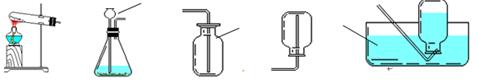
�����������1��ͼ�������ܢݵ����Ƣ��ձ����ݾƾ��ơ�
��2��ʵ������ȡ������ijͬѧ�����ͼA��װ�ã����ǹ����Һ�巴Ӧ����Ҫ���ȵ����ͣ������������ռ����������У���������ʢ�ŵ��Լ�ӦΪ����������Һ���˷����������Ļ�ѧ����ʽΪ2H2O2
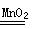 2H20+O2��
2H20+O2���������ˮ���ռ������� A�м���ƿ�еĵ���ӦΪ��ͼ��ʾ��

��3��ʵ������ȡ������̼����B~G��ѡ����������װ����װ�ã�Ҫ���ܷ�����Ʒ�Ӧ�ķ�����ֹͣ����ȷ��ѡ��ΪCEFG����Ӧ�Ļ�ѧ����ʽΪCaCO3+2HCl===CaCl2+CO2 + H2O��
��4��ʵ����������ͼ��ʾ��ϴ��װ�ÿ����ڼ��������̼���壬ϴ��ƿ������װ��ҩƷ�����ǣ�A��������ʯ��ˮ���йط�Ӧ�Ļ�ѧ����ʽΪCO2+ Ca(OH)2=CaCO3+ H2O��
��5����ѡ����ͼװ�����ռ�CO2��������Ӧ��a���롣
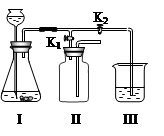
������ʵ������ȡ���壬ʹ�õ�ʵ��װ�ã�Ҫ���������������������������ʡ���Ӧ���״̬��������ѡ������̼��ʹ����ʯ��ˮ����ǣ��˷�Ӧ�����������������̼��

��ϰ��ϵ�д�
�����Ŀ