��Ŀ����
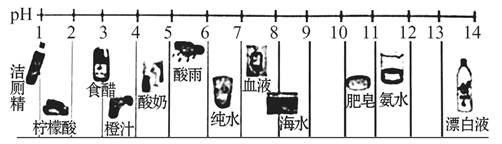
2013����ʡ���ֵ���������ˮƽ��pH���±�����ش��������⣺
��1������pH��ֽ�ⶨ��ˮ�����ȣ��ⶨ�����ǣ� ����2�֣�
��2���ϱ��оٵ�7�������У�û��������Ⱦ�ĵ�����_______�������������Ҫ������_________�������)�� ��CO ��CO2 ��SO2 ��NO2��
��3�����������Ҫ�ɷ�ΪH2SO4��HNO3����д�����л�ѧ����ʽ��
(a)��H2SO4�����긯ʴ�����ҵ�ʯ��ʯ ��
(b)��HNO3��������������������ʯ�ҷ�Ӧ ��
| ���� | ���� | ���� | ��ɽ | ��Դ | ���� | ��ݸ | ��ɽ |
| 2008����ˮƽ��pH | 4��47 | 4��8 | 4��54 | 6��37 | 4��86 | 4��83 | 4��58 |
��1������pH��ֽ�ⶨ��ˮ�����ȣ��ⶨ�����ǣ� ����2�֣�
��2���ϱ��оٵ�7�������У�û��������Ⱦ�ĵ�����_______�������������Ҫ������_________�������)�� ��CO ��CO2 ��SO2 ��NO2��
��3�����������Ҫ�ɷ�ΪH2SO4��HNO3����д�����л�ѧ����ʽ��
(a)��H2SO4�����긯ʴ�����ҵ�ʯ��ʯ ��
(b)��HNO3��������������������ʯ�ҷ�Ӧ ��
��1����PH��ֽ���ڲ���Ƭ�ϣ��������Һ�壬�����ɫ���Ƚϣ��ó�PHֵ
��2����Դ �ۢ�
��3��(a��CaCO3+H2SO4=CaSO4+H2O+CO2 (b��Ca(OH)2+2HNO3=Ca(NO3)2+2H2O
��4����
��2����Դ �ۢ�
��3��(a��CaCO3+H2SO4=CaSO4+H2O+CO2 (b��Ca(OH)2+2HNO3=Ca(NO3)2+2H2O
��4����
�����������1����Һ���ȵIJⶨ�����ǣ���PH��ֽ���ڲ���Ƭ�ϣ��������Һ�壬�����ɫ���Ƚϣ�
��2�������PHС��5��6��ֻ�к�Դû��������Ⱦ������������Ҫԭ���ǻ�ʯȼ��ȼ�ղ����Ķ�������
��3��ʯ��ʯ����Ҫ�ɷ���̼��ƣ�����������ֽⷴӦ�������������кͷ�Ӧ�����ɵ����κ�ˮ��

��ϰ��ϵ�д�
�����Ŀ
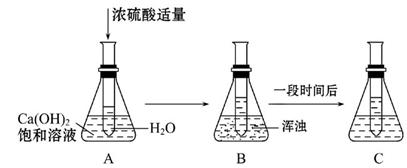
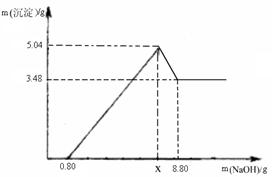
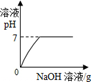
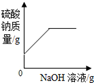
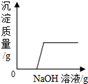
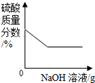
 �������仯������û���漰���Ļ�ѧ��Ӧ������
�������仯������û���漰���Ļ�ѧ��Ӧ������