题目内容
【题目】某化学兴趣小组用一氧化碳与氧化铁的反应来探究炼铁的原理,装置如下图所示。
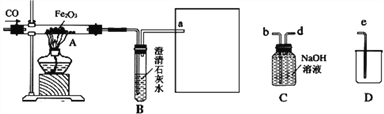
(1)为避免一氧化碳污染空气,并回收利用一氧化碳,方框中连接的是C和D,导管接口的连接顺序为a→(_________)→ (_________)→(_________)。除此方法外还可以用_____________或____________的方法处理尾气。
(2)实验开始时,应____________(填“先加热再通CO”或“先通CO再加热”),目的是___________。
(3)实验进行一段时间后,玻璃管A中出现的现象为_____________,反应的化学方程式为______________。
(4) B装置的作用是___________________,方程式是_________________________。
【答案】 d b e 点燃 收集 先通CO再加热 排出玻璃管中的空气,防止加热时发生爆炸 红色粉末逐渐变为黑色 3CO + Fe2O3 高温 2Fe + 3CO2 校验生成的CO2 CO2 + Ca(OH)2 === CaCO3↓+ H2O
【解析】(1)为避免一氧化碳污染空气,并回收利用一氧化碳,方框中连接的是C和D,收集一氧化碳,因一氧化碳排到空气中污染空气,导管接口的连接顺序为a→d→ b→c,才能达到吸收二氧化碳、收集一氧化碳的目的。除此方法外还可以用点燃或直接用气囊收集尾气的方法处理尾气; (2)实验开始时,应先通CO再加热,目的是排出玻璃管中的空气,防止加热时发生爆炸;(3)实验进行一段时间后,玻璃管A中出现的现象为红色粉末逐渐变为黑色,反应的化学方程式为:3CO + Fe2O3 高温 2Fe + 3CO2;(4) B装置的作用是验证并收集二氧化碳,方程式是CO2 + Ca(OH)2 === CaCO3↓+ H2O。

 名校课堂系列答案
名校课堂系列答案【题目】小君在家自制奶香馒头。
原料:面粉(中筋粉)4杯、酵母1匙、白糖3大匙、纯牛奶80毫升、水140毫升、奶粉3大匙、小苏打1匙、食盐3分之1小匙。

步骤如下:和面、搓条、成形、发酵、蒸制。
(1)小君取140毫升水,家里没有量筒或量杯,只找到妹妹用的三个不同规格的奶瓶。上图最适合量取140毫升水的是_________
A. 50mL奶瓶 B. 150mL奶瓶 C.250mL奶瓶
(2)奶粉和纯牛奶中富含人类必需的6大营养素中的_____________。
(3)小苏打在2700C时,完全分解转化成苏打、二氧化碳和水。二氧化碳能使馒头疏松、多孔、口感好。写出化学方程式_____________________________________。
(4)小君请教化学老师设计了一个模拟实验证明白色粉末里有纯碱和食盐。完成下表。限选试剂:氯化钡溶液、硝酸银溶液、稀盐酸、稀硝酸、澄清石灰水、氯化钙溶液
实验操作 | 预期现象和结论 |
步骤1:取少量白色粉末于烧杯中,_______________ | 白色粉末溶解,形成无色溶液 |
步骤2:取步骤1形成的溶液与试管中,再__________________________ | _______________________,说明样品中含有纯碱 |
步骤3:____________________ | _______________,说明样品中含有食盐 |