��Ŀ����
����Ŀ��̼�ڵؿ��еĺ������ߣ������Ļ����������ڶ࣬���ҷֲ����㣮
������ѧ֪ʶ�ش�
��1��������̼�Ļ�ѧ�����ȶ���ԭ��
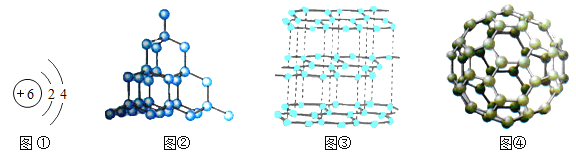
��2��ͼ��������ͼ�������������ʲ���ͬ��ԭ����
3CΪ+4�ۣ�ClΪ-1�ۣ���C��Cl��ɵĻ�����Ļ�ѧʽΪ
4X��Y��Z�dz��л�ѧ���������ʣ�����֮������ͼ��ʾ��ת����ϵ��X���ж����壬Y�Dz�֧��ȼ�յ����壬��ش��������⡣
![]()
�� X�Ļ�ѧʽΪ___________��
��Y��ʯ��ˮ��Ӧ����Z�Ļ�ѧ����ʽΪ
������X��Y������Ԫ����ͬ�������ǵĻ�ѧ���ʲ�ͬ����ԭ���� ��
5ʵ�������ô���ʯ��ϡ���ᷴӦ��ȡ������̼ ����Ҫ��ȡ44��������̼���塣���㣺Ҫ��̼���80%�Ĵ���ʯ���ٿˣ�
���𰸡���1��̼�Ȳ����õ�����Ҳ������ʧȥ����
��2��ԭ�ӵ����з�ʽ��ͬ��3��CCl4
��4����CO��Ca��OH��2+CO2===H2O+CaCO3�������ӵĹ��ɲ�ͬ��5��125��
��������
���������������̼�Ļ�ѧ�����ȶ���ԭ��̼�Ȳ����õ�����Ҳ������ʧȥ���ӣ�ͼ��������ͼ�������������ʲ���ͬ��ԭ����ԭ�ӵ����з�ʽ��ͬ��CΪ+4�ۣ�ClΪ-1�ۣ���C��Cl��ɵĻ�����Ļ�ѧʽΪCCl4��X���ж����壬Y�Dz�֧��ȼ�յ����壬˵��X��CO����Y�Ƕ�����̼��Y��ʯ��ˮ��Ӧ����Z�Ļ�ѧ����ʽΪCa��OH��2+CO2===H2O+CaCO3���� ������X��Y������Ԫ����ͬ�������ǵĻ�ѧ���ʲ�ͬ����ԭ�������ӵĹ��ɲ�ͬ��
��Ҫ��̼���80%�Ĵ���ʯX��
CaCO3 + 2HCl==CaCl2 + CO2�� + H2O
100 44
80%X 44��
�б���ʽ�ã�100:80%X=44:44�� ���x=1245��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�