��Ŀ����
����Ŀ��(1)���Ƽݵ��ظ����(K2Cr2O7) �и�Ԫ�صĻ��ϼ�Ϊ_____________��
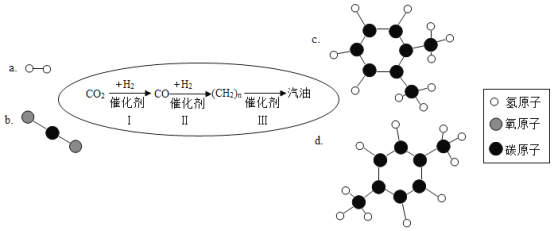
(2)��6�����������Ļ�ѧʽΪ__________, ����__________________��
(3)���з�̪��NaOH��Һ�еμ����ᣬ��Һ��ɫ��ȥ��Ϊ��ɫ����ʱ��ҺpH___________7
(4)Ҫ����6%��NaCl��Һ50g.��Ҫ����NaCl____________g,ˮ__________mL (ˮ���ܶ�Ϊ1g/cm3)
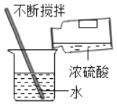
(5)��50g 98%��Ũ��������450g ˮ�У�������Һ�����ʵ���������Ϊ______________��
���𰸡�+6 SO3 �������� ![]() 3 47 9.8%
3 47 9.8%
��������
(1)���Ƽݵ��ظ����(K2Cr2O7) �У����Ԫ�صĻ��ϼ�ΪX,���ݻ�������Ԫ�ػ��ϼ۵Ĵ�����Ϊ0��![]() ����+1����2+2X+��-2����7=0��X=+6�����+6��
����+1����2+2X+��-2����7=0��X=+6�����+6��
(2)��6������������У���Ԫ��Ϊ-2�ۣ����ݻ������и�Ԫ�ػ��ϼ۵Ĵ�����Ϊ�㣬�����������Ļ�ѧʽΪSO3�� �������������SO3����������
(3)��̪�����죬�����Ժ�������Һ����ɫ�����з�̪��NaOH��Һ�����ԣ��еμ����ᣨ���ԣ�����Һ��ɫ��ȥ��Ϊ��ɫ���п�����������������ǡ�÷�Ӧ����Һ�����ԣ�pH=7��Ҳ�п��������������Һ������pH<7�����pH�� 7��
(4)Ҫ����6%��NaCl��Һ50g�����Լ������Ҫ����NaCl������Ϊ![]() ����Ҫ��ˮ�����Ϊ
����Ҫ��ˮ�����Ϊ![]() �����3��47��
�����3��47��
(5)��50g 98%��Ũ��������450g ˮ�У���������Һ�����ʵ���������ΪX������ϡ��ǰ������������������ɵã�![]() ����ã�X=9.8%��
����ã�X=9.8%��
��������Һ�����ʵ���������Ϊ9.8%�����9.8%��

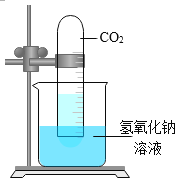
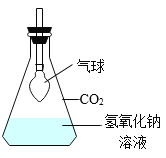
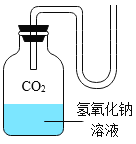
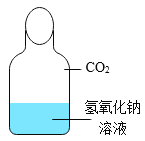
����Ŀ����ѧ��һ����ʵ��Ϊ������ѧ�ƣ���ͼ��һ��̽�� CO2 ��NaOH �ܷ�Ӧ��ʵ�顣
ʵ�� | A | B | C | D | E |
ʵ��װ�� |
|
|
|
|
�����켦�� |
��1������д��Ӧ��ʵ������A______________�� B_____________�� C_______________�� D_____________�� E_______________��
��2����ͼ 1 װ�ý���ʵ�飬�Ⱥ� 2 ��ע�����е���Һ����ȫ�����룬���һ��ʱ����װ����ѹǿ�仯��ͼ 2 ��ʾ�����������Һ��____________��
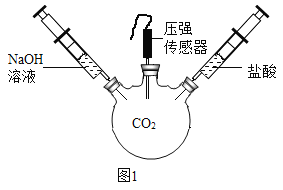
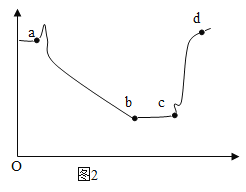
��3��ab ��ѹǿ����仯��������ͼ��ʾ��ԭ���ǣ�д��Ӧ�Ļ�ѧ����ʽ����_______________��
��4��bc ��ѹǿ���䣬ԭ����_______________��
��5��cd ��ѹǿ�����û�ѧ����ʽ����_________________��