��Ŀ����
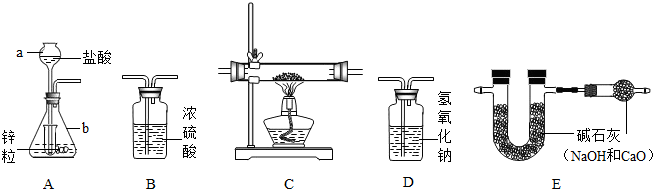
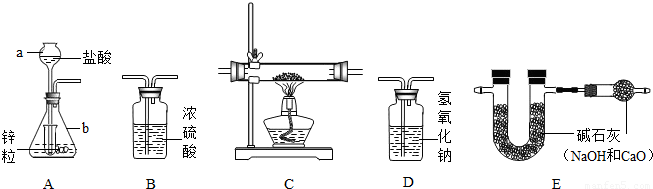
Ϊ�˲ⶨijͭ������ͭ�Ļ����������ͭ������������ij�о���С�����������ʵ��װ�� ��װ��C����װ����ҩƷΪͭ������ͭ�Ļ������Իش�������⣺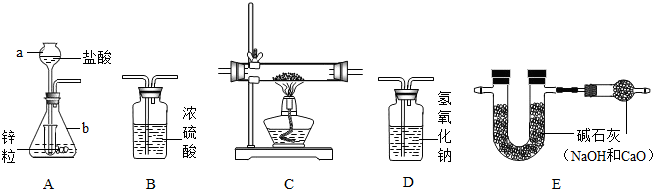
��1��д������������ƣ�a
��2����ȷ��װ������˳��
��3��Aװ����С�Թܵ�����
��4�����û��Dװ�ã�Ϊ�˲�Ӱ��ⶨ������ɽ�A�е����ỻ��
��5����ʵ��ǰ����ͭ������ͭ�Ļ��������Ϊ2�ˣ�ʵ�����װ��E��U�ܵ�����������0.36�ˣ����ͭ������ͭ�Ļ����������ͭ����������Ϊ

��1��д������������ƣ�a
����©��
����©��
��b��ƿ
��ƿ
����2����ȷ��װ������˳��
ADBCE
ADBCE
����дװ����ţ�����3��Aװ����С�Թܵ�����
Һ�⡢��ֹ����������ӳ���©����ɢ�������С���ԼҩƷ��
Һ�⡢��ֹ����������ӳ���©����ɢ�������С���ԼҩƷ��
����4�����û��Dװ�ã�Ϊ�˲�Ӱ��ⶨ������ɽ�A�е����ỻ��
ϡ����
ϡ����
����5����ʵ��ǰ����ͭ������ͭ�Ļ��������Ϊ2�ˣ�ʵ�����װ��E��U�ܵ�����������0.36�ˣ����ͭ������ͭ�Ļ����������ͭ����������Ϊ
80%
80%
����������1������������;�����
��2��ʵ��������п��ϡ������ȡ����ʱ���������ӷ��������Ƶõ������ڻ�������HCl�����ˮ���������õ������������H2���������HCl�����ˮ������
��3�����ݳ�����©����嵽Һ�����£���ƿ������ϴ����
��4������������������ʵIJ�ͬ�����
��5������ˮ�������������ͭ�������������������ͭ������������
��2��ʵ��������п��ϡ������ȡ����ʱ���������ӷ��������Ƶõ������ڻ�������HCl�����ˮ���������õ������������H2���������HCl�����ˮ������
��3�����ݳ�����©����嵽Һ�����£���ƿ������ϴ����
��4������������������ʵIJ�ͬ�����
��5������ˮ�������������ͭ�������������������ͭ������������
����⣺��1������������;д�����ƣ�a�dz���©����b����ƿ
��2������ÿ��װ�õ����ã�ʵ��������п��ϡ������ȡ����ʱ���������ӷ��������Ƶõ������ڻ�������HCl�����ˮ���������õ������������H2����������װ��D����HCl��������װ��B����ˮ��������Cװ�û�ԭ����ͭ����E��֤���ɵ�ˮ������ˮ��������˳��ΪADBCE
��3��Aװ���г���©����嵽Һ�����£���ƿ������ϴ���������϶࣬���С�Թܵ�������Һ�⡢��ֹ����������ӳ���©����ɢ�������С���ԼҩƷ��
��4��װ��D��ʢ��������������Һ����������HCl����ģ���û��װ��D���ɽ����ỻ��ϡ����
��5��U�����ӵ�����Ϊ���ɵ�ˮ�����������������ԭ����ͭ���ɵ�ˮ������Ϊ0.36�ˣ�
������ͭ������Ϊx
H2+CuO
Cu+H2O
80 18
x 0.36g
=
x=1.6g
����ͭ����������Ϊ
��100%=80%
�ʴ�Ϊ����1������©������ƿ��2��ADBCE��3��Һ�⡢��ֹ����������ӳ���©����ɢ�������С���ԼҩƷ��
��4��ϡ���ᣨ5��80%
��2������ÿ��װ�õ����ã�ʵ��������п��ϡ������ȡ����ʱ���������ӷ��������Ƶõ������ڻ�������HCl�����ˮ���������õ������������H2����������װ��D����HCl��������װ��B����ˮ��������Cװ�û�ԭ����ͭ����E��֤���ɵ�ˮ������ˮ��������˳��ΪADBCE
��3��Aװ���г���©����嵽Һ�����£���ƿ������ϴ���������϶࣬���С�Թܵ�������Һ�⡢��ֹ����������ӳ���©����ɢ�������С���ԼҩƷ��
��4��װ��D��ʢ��������������Һ����������HCl����ģ���û��װ��D���ɽ����ỻ��ϡ����
��5��U�����ӵ�����Ϊ���ɵ�ˮ�����������������ԭ����ͭ���ɵ�ˮ������Ϊ0.36�ˣ�
������ͭ������Ϊx
H2+CuO
| ||
80 18
x 0.36g
| 80 |
| x |
| 18 |
| 0.36g |
����ͭ����������Ϊ
| 1.6g |
| 2g |
�ʴ�Ϊ����1������©������ƿ��2��ADBCE��3��Һ�⡢��ֹ����������ӳ���©����ɢ�������С���ԼҩƷ��
��4��ϡ���ᣨ5��80%
������������Ҫ����������ԭ����ͭ���Լ����ݷ�Ӧǰ�����������仯�������ͭ�������ȷ����֪ʶ����һ��ʵ����������ϵ�̽���⣻

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ