��Ŀ����
��2008?������һģ����ͼΪʵ�����о������Ļ�ѧԭ����װ�ã���ش��ʵ���йص����⣮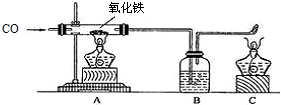
��1��ʵ�鿪ʼʱӦ��ͨ��
��2��Bƿ��װ�г���ʯ��ˮ��������Ϊ
��3��C���ƾ��Ƶ�����
��4�����к����ʵ���������Ʒ10�ˣ����ʲ��μӷ�Ӧ����Ϊ�ⶨ����Ʒ��������������������ijͬѧ����ͼ��ʾ��װ�ý���ʵ�飬�õ���������ʵ�����ݣ�
����ΪӦѡ��

��1��ʵ�鿪ʼʱӦ��ͨ��
������һ����̼
������һ����̼
����2��Bƿ��װ�г���ʯ��ˮ��������Ϊ
��֤��CO2����
��֤��CO2����
����Ӧ�Ļ�ѧ����ʽΪCa��OH��2+CO2=CaCO3��+H2O
Ca��OH��2+CO2=CaCO3��+H2O
����3��C���ƾ��Ƶ�����
�����
�����
����4�����к����ʵ���������Ʒ10�ˣ����ʲ��μӷ�Ӧ����Ϊ�ⶨ����Ʒ��������������������ijͬѧ����ͼ��ʾ��װ�ý���ʵ�飬�õ���������ʵ�����ݣ�
| ��Ӧǰ | ��������ȫ��Ӧ�� | |
| ���� | ϴ��ƿ��ʯ��ˮ������Ϊ190g | ϴ��ƿ��ƿ�����ʵ�������Ϊ196g |
| ���� | �����ܺ���������Ʒ������Ϊ57.9g | �����ܺ��ڹ���������Ϊ55.2g |
��
��
���������������Ʒ������������������������Ϊ90%
90%
���������������е�֪ʶ���з�����һ����̼���ڿ�ȼ�����壬����ʱ���ȿ��ܲ�����ը��������̼���ó���ʯ��ˮ���飬һ����̼���ж��ԣ���Ҫ����β������������ʹ��ȼ�շ���Ҫ�����������ĺ���������������������ݣ����п��ܶ�����̼�����ղ���ȫ���¼���Ľ����ȷ��
����⣺��1��ʵ�鿪ʼʱҪͨ��������һ����̼���ž�װ���еĿ�������ֹ����ʱ������ը�����������һ����̼��
��2������ʯ��ˮ���������������̼�Ĵ��ڣ������������������̼��Ӧ����̼��ƺ�ˮ�������֤��CO2���ɣ�Ca��OH��2+CO2=CaCO3��+H2O��
��3��һ����̼�ж�����Ҫȼ�մ��������β��������
��4���������ʯ��ˮ���ն�����̼������������յIJ���ȫ����������ȷ����ѡ�����飬���ݱ����ṩ�����ݿ��Կ����������ܼ���������ļ�����Ϊ��57.9g-55.2g=2.7g����������������Ϊx
Fe2O3+3CO
2Fe+3CO2��m
160 112 48
x 2.7g
=
x=9g
��Ʒ������������������Ϊ��
��100%=90%
����ң�90%��
��2������ʯ��ˮ���������������̼�Ĵ��ڣ������������������̼��Ӧ����̼��ƺ�ˮ�������֤��CO2���ɣ�Ca��OH��2+CO2=CaCO3��+H2O��
��3��һ����̼�ж�����Ҫȼ�մ��������β��������
��4���������ʯ��ˮ���ն�����̼������������յIJ���ȫ����������ȷ����ѡ�����飬���ݱ����ṩ�����ݿ��Կ����������ܼ���������ļ�����Ϊ��57.9g-55.2g=2.7g����������������Ϊx
Fe2O3+3CO
| ||
160 112 48
x 2.7g
| 160 |
| x |
| 48 |
| 2.7g |
x=9g
��Ʒ������������������Ϊ��
| 9g |
| 10g |
����ң�90%��
���������⿼����һ����̼��ԭ��������ʵ���Լ������������ĺ�������ɴ��⣬�����������ʵ����ʽ��У�

��ϰ��ϵ�д�
�����Ŀ
 ��2008?������һģ��ȼ�������ǵ����������ķ�չ�������е���ϵ��
��2008?������һģ��ȼ�������ǵ����������ķ�չ�������е���ϵ��