题目内容
(2005?汕头)某同学为探究铁合金中铁的质量分数,先后进行了三次实验,实验数据如下表:| 实验次数 项目 | 第一次 | 第二次 | 第三次 |
| 所取合金的质量/g | 20 | 20 | 40 |
| 所加稀硫酸的质量/g | 100 | 120 | 80 |
| 生成氢气的质量/g | 0.4 | 0.4 | 0.4 |
(1)上表三次实验中,合金里的铁恰好完全反应时,消耗稀硫酸溶液的质量是______g.
(2)该铜铁合金中铁的质量分数是多少?______;
(3)第三次实验所得溶液溶质质量分数为______(结果保留至0.1%).
【答案】分析:(1)先对比分析第一次和第二次的实验数据,可以分析出要产生0.4g的氢气需要合金20g,所以在第三次试验中产生的0.4g氢气是20g合金与80g酸生成的;
(2)假设合金中铁的质量分数为x,则20g合金中铁的质量为20x,再根据铁与稀硫酸反应的方程式进行相关计算即可;
(3)第三次实验中的溶质是FeSO4,FeSO4的质量可以根据(2)中的计算结果求出,为30.4g,FeSO4溶液的质量=铁的质量+硫酸的质量-生成氢气的质量.
解答:解:(1)第一次和第二次两个实验所加合金质量相同,而所加稀硫酸的质量不同,但最后产生氢气的质量相同,说明第一次和第二次两个实验中合金均反应完,第二次实验中的酸一定过量;
第三次实验与前两次实验相比,合金质量加倍,而稀硫酸的质量减少,但产生氢气质量不变,所以20g合金反应完需要消耗稀硫酸80g;
故答案为:80g;
(2)设合金中铁的质量分数为x,
Fe+H2SO4=FeSO4+H2↑
56 2
20x 0.4
可得: =
=
解得:x=56%
故答案为:56%;
(3)设生成FeSO4的质量为y,
Fe+H2SO4=FeSO4+H2↑
56 152
20×56% y
可得: =
=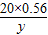
解得y=30.4g
所得FeSO4溶液的质量=20×56%+80-0.4=90.8g
所以第三次实验所得溶液溶质质量分数为 100%=33.5%
100%=33.5%
故答案为:33.5%
点评:虽然溶液的质量=溶剂的质量+溶质的质量,但有时用这种方法计算溶液的质量会很复杂,这时采用整体法去计算溶液的质量会比较简单.
(2)假设合金中铁的质量分数为x,则20g合金中铁的质量为20x,再根据铁与稀硫酸反应的方程式进行相关计算即可;
(3)第三次实验中的溶质是FeSO4,FeSO4的质量可以根据(2)中的计算结果求出,为30.4g,FeSO4溶液的质量=铁的质量+硫酸的质量-生成氢气的质量.
解答:解:(1)第一次和第二次两个实验所加合金质量相同,而所加稀硫酸的质量不同,但最后产生氢气的质量相同,说明第一次和第二次两个实验中合金均反应完,第二次实验中的酸一定过量;
第三次实验与前两次实验相比,合金质量加倍,而稀硫酸的质量减少,但产生氢气质量不变,所以20g合金反应完需要消耗稀硫酸80g;
故答案为:80g;
(2)设合金中铁的质量分数为x,
Fe+H2SO4=FeSO4+H2↑
56 2
20x 0.4
可得:
 =
=
解得:x=56%
故答案为:56%;
(3)设生成FeSO4的质量为y,
Fe+H2SO4=FeSO4+H2↑
56 152
20×56% y
可得:
 =
=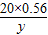
解得y=30.4g
所得FeSO4溶液的质量=20×56%+80-0.4=90.8g
所以第三次实验所得溶液溶质质量分数为
 100%=33.5%
100%=33.5%故答案为:33.5%
点评:虽然溶液的质量=溶剂的质量+溶质的质量,但有时用这种方法计算溶液的质量会很复杂,这时采用整体法去计算溶液的质量会比较简单.

练习册系列答案
 阅读快车系列答案
阅读快车系列答案
相关题目
(2005?汕头)某同学为探究铁合金中铁的质量分数,先后进行了三次实验,实验数据如下表:
根据该同学的实验,试回答以下问题:
(1)上表三次实验中,合金里的铁恰好完全反应时,消耗稀硫酸溶液的质量是______g.
(2)该铜铁合金中铁的质量分数是多少?______;
(3)第三次实验所得溶液溶质质量分数为______(结果保留至0.1%).
| 实验次数 项目 | 第一次 | 第二次 | 第三次 |
| 所取合金的质量/g | 20 | 20 | 40 |
| 所加稀硫酸的质量/g | 100 | 120 | 80 |
| 生成氢气的质量/g | 0.4 | 0.4 | 0.4 |
(1)上表三次实验中,合金里的铁恰好完全反应时,消耗稀硫酸溶液的质量是______g.
(2)该铜铁合金中铁的质量分数是多少?______;
(3)第三次实验所得溶液溶质质量分数为______(结果保留至0.1%).
(2005?汕头)某同学为探究铁合金中铁的质量分数,先后进行了三次实验,实验数据如下表:
根据该同学的实验,试回答以下问题:
(1)上表三次实验中,合金里的铁恰好完全反应时,消耗稀硫酸溶液的质量是______g.
(2)该铜铁合金中铁的质量分数是多少?______;
(3)第三次实验所得溶液溶质质量分数为______(结果保留至0.1%).
| 实验次数 项目 | 第一次 | 第二次 | 第三次 |
| 所取合金的质量/g | 20 | 20 | 40 |
| 所加稀硫酸的质量/g | 100 | 120 | 80 |
| 生成氢气的质量/g | 0.4 | 0.4 | 0.4 |
(1)上表三次实验中,合金里的铁恰好完全反应时,消耗稀硫酸溶液的质量是______g.
(2)该铜铁合金中铁的质量分数是多少?______;
(3)第三次实验所得溶液溶质质量分数为______(结果保留至0.1%).
22.(2005?汕头)某同学为探究铁合金中铁的质量分数,先后进行了三次实验,实验数据如下表:
根据该同学的实验,试回答以下问题:
(1)上表三次实验中,合金里的铁恰好完全反应时,消耗稀硫酸溶液的质量是______g.
(2)该铜铁合金中铁的质量分数是多少?______;
(3)第三次实验所得溶液溶质质量分数为______(结果保留至0.1%).
| 实验次数 项目 | 第一次 | 第二次 | 第三次 |
| 所取合金的质量/g | 20 | 20 | 40 |
| 所加稀硫酸的质量/g | 100 | 120 | 80 |
| 生成氢气的质量/g | 0.4 | 0.4 | 0.4 |
(1)上表三次实验中,合金里的铁恰好完全反应时,消耗稀硫酸溶液的质量是______g.
(2)该铜铁合金中铁的质量分数是多少?______;
(3)第三次实验所得溶液溶质质量分数为______(结果保留至0.1%).