��Ŀ����
����Ŀ�����ᾧ��Ļ�ѧʽΪH2C2O4��2H2O�����ᾧ��100�濪ʼʧˮ��101.5���ۻ���150�����ҷֽ⣻���������ڵ����¿�����Ϊ���壻�����Ƕ�Ԫ�ᣬ��������κͼ���������ˮ���������������ˮ���������������ṩ����Ϣ���ش��������⣺
��1�����ü��Ȳ��ᾧ��ķ�����ȡijЩ���壬Ӧ��ѡ������巢��װ���ǣ�ͼ�м���װ������ȥ����______��
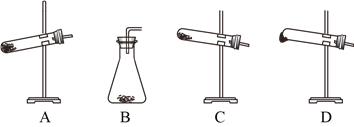
��2��ijС��ͬѧ����������ȷֽ������CO��CO2���������̽��ʵ�飬��������װ�����һ����֤�����ֲ����ʵ��װ�ã����ᾧ��ֽ�װ���ԣ�װ�ò����ظ�ʹ�ã���



������װ��˳��______________________________________________________��
��װ��A��������______________________________________________________��
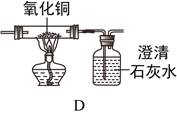
�ۼ����������CO��ʵ��������_________________________________________��
��3����С��ͬѧ���������ữ��KMnO4��Һ�е�������IJ�����Һ����������KMnO4��Һ��ɫ���Ӷ��жϲ�����н�ǿ�Ļ�ԭ�ԡ�����ɸ÷�Ӧ�Ļ�ѧ����ʽ��___________��
2KMnO4+5H2C2O4+��__________=2MnSO4+K2SO4+��__________+��H2O
���𰸡� D A��B��D��C ��������ȥ�����ᣬ��ֹ���Ŷ�����̼�ļ��飨���ֹ�������Bװ�ã����������� D�к�ɫ�����ɺ�ɫ���ҳ���ʯ��ˮ����� 2KMnO4+5H2C2O4+3 H2SO4=2MnSO4+K2SO4+10 CO2��+8 H2O
�������������ڲ���ֽ���龳�¿����˷���װ�õ�ѡ��һ����̼�Ķ�����̼�ļ����Լ���ѧ����ʽ����ƽ�����ݲ������ۻ����ص�ѡ����װ�ã�����һ����̼�Ķ�����̼���Խ���װ���Ⱥ�˳���ѡ���������غ㶨�ɽ���Σ��Ʒ����ʽ����ƽ��
��1�����ᾧ��100�濪ʼʧˮ��101.5���ۻ���150�����ҷֽ⣬���Լ��Ȳ��ᾧ��ķ�����ȡijЩ���岻��ѡ��A��B��C����ѡD��
��2���ټ��Ȳ��ᾧ��ʱ���ܲ����������������������ڵ����¿�����Ϊ���������ñ�ˮ������ȥ����������Ȼ���ó���ʯ��ˮ���������̼�����ü��ȵ�CuO�ͳ���ʯ��ˮ����CO������ռ�β������������������˳��Ϊ��A��B��D��C��
�ڼ��Ȳ��ᾧ��ʱ���ܲ����������������������ڵ����¿�����Ϊ���壬װ��A����������������ȥ�����ᣬ��ֹ���Ŷ�����̼�ļ��飨���ֹ�������Bװ�ã�������������
��һ����̼��������ͭ��Ӧ���ɶ�����̼��ͭ��������̼��ʹ����ʯ��ˮ����ǣ����Լ����������CO��ʵ��������D�к�ɫ�����ɺ�ɫ���ҳ���ʯ��ˮ����ǣ�
��3�����������غ㶨�ɣ���ѧ��Ӧǰ��ԭ�ӵ������ԭ�ӵ���Ŀ���䣬��ѧ����ʽ��ƽ���£�2KMnO4+5H2C2O4+3 H2SO4=2MnSO4+K2SO4+10 CO2��+8 H2O��