��Ŀ����
ͨ��һ���ѧϰ����������ѧ���Ļ�ѧ֪ʶ����������⣺
(1) �·��ϵ����ۿ���������ϴ������ԭ����_______________________________��
Ҳ�����ü���ϴ�Ӽ���ˮϴ������ԭ����______________________________��
(2) θ�����IJ��ˣ������ú���������þ��ҩ���Լ��Სʹ���䷴Ӧ�Ļ�ѧ����ʽ
Ϊ________________________________________��
��3��ʹ�ܽ���ˮ�еĿ����ݳ����Բ���__________��С�����ܽ�ȵķ�����
��4����ϡ������ϴ������������⣨��Ҫ�ɷ���Fe2O3�����䷴Ӧ�Ļ�ѧ����ʽΪ�� ���÷�Ӧ���ڻ�����Ӧ�����е� ��Ӧ��
��5�����ʯ��ˮ���ձ����������γɰ�ɫ�Ĺ������ʣ���Ҫ����ϡ������ϴ���䷴Ӧ�Ļ�ѧ����ʽΪ�� ��
(1�����Ϳ����ܽ����ۣ�ϴ�Ӽ����Խ������黯
��2��![]()
��3�������¶Ȼ��Сѹǿ
�� ��4�� Fe2O3+6HCl=2FeCl3+3H2O���� ����
��5��CaCO3 +2HCl��![]() �� CaCl2 + CO2��+ H2O
�� CaCl2 + CO2��+ H2O

��ϰ��ϵ�д�
�����Ŀ
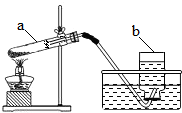 ����ͼװ�ü��ȸ��������ȡ��������ش�
����ͼװ�ü��ȸ��������ȡ��������ش�
 ͨ��һ���ѧϰ�����dz�ָ��ܵ��˻�ѧʵ���еġ�ɫ����������ͼ��ʾ����֪A��Һ��һ����ʹ��ɫʯ����Һ������ɫҺ�壬A��Һ��һ�������¿ɷ�������ת�䣺
ͨ��һ���ѧϰ�����dz�ָ��ܵ��˻�ѧʵ���еġ�ɫ����������ͼ��ʾ����֪A��Һ��һ����ʹ��ɫʯ����Һ������ɫҺ�壬A��Һ��һ�������¿ɷ�������ת�䣺