��Ŀ����
����Ŀ����һ���ѷ���һ����װ̼����(NH4HCO3)���ʵ�С�ֿ���,����һ������,����Ա���ֲֿ������ֻ�ѧ�����еĴ̼�����ζ���Ũ���ˡ���Щ����Ļ��ʱ����ˡ���鷢�ֱ����ˡ���鷢��:���ٵĻ��ʰ��b��������������.��û�з��ֻ��ʾ����ڵ��ϡ���ѧ�С��Ϊ��̽�����ʱ��ٵ�ԭ��������Լ��IJ��벢�������ͼ��ʵ�顣���ǵ�ʵ���¼���±�:
ʵ�鲽�� | ʵ������ |
ȡ����̼����粒����ĩ�����������У�������������ھƾ����ϼ��� | �ٰ�ɫ��������ʧ;��____ |

(1)̼��������Ⱥ�ֽ�,����ʵ������ٵ�ԭ����___,�����ǵIJ�����ȷ,ʵ�������ӦΪ___
(2)д���÷�Ӧ�Ļ�ѧ����ʽ___
(3)̼�����Ӧ��α���?____
(4)ij�����������Ⱥ�仯Ϊ̬���ֱ仯������_________��
A һ���������仯
B һ���ǻ�ѧ�仯
C �����������仯,Ҳ�����ǻ�ѧ�仯
D �Ȳ��������仯Ҳ���ǻ�ѧ�仯
(5)��֪���������ԭ��������: H-I,C-12,N-I4,O-16ͨ������д�����
��̼����淋���Է�������Ϊ:____
�ڸ�Ԫ��������Ϊ:_____
�۵�Ԫ�ص���������Ϊ(������0.001 ):____
���𰸡��ֽ�����ж�����̼��������ˮ�����ж�����̼�백������,ˮ���Ⱥ�����ˮ������������ɢ�������� �д̼�����ζ NH4HCO3![]() NH3��+CO2��+H2O �����������ɱ����ܷⱣ�� C 79 14��5��12��48 17.7%
NH3��+CO2��+H2O �����������ɱ����ܷⱣ�� C 79 14��5��12��48 17.7%
��������
��1��̼��������Ⱥ�ֽ�����ж�����̼��������ˮ�����ж�����̼�백�������壬ˮ���Ⱥ�����ˮ������������ɢ�������У�ʵ�������ӦΪ�д̼�����ζ�����������
��2��̼��������Ⱥ�ֽ�����ж�����̼��������ˮ���÷�Ӧ�Ļ�ѧ����ʽNH4HCO3![]() NH3��+CO2��+H2O��
NH3��+CO2��+H2O��
��3��̼���������ʱ�ֽ⣬��Ӧ�ܷⱣ���ҷ�����������
��4�� �����������ȱ�Ϊ���壬���ֱ仯�����������仯��Ҳ�����ǻ�ѧ�仯������̼��������ȷֽ����ڻ�ѧ�仯�������ȱ��ˮ�������������仯��
��5�� ��̼����淋���Է�������=14+1��4+1+12+16��3=79��
�ڸ���̼����淋Ļ�ѧʽ��֪��������N��H��C��O����Ԫ�أ���Ԫ�ص�������Ϊ14����1��5����12����16��3��=14��5��12��48��
��̼������е�Ԫ�ص���������=![]() ��100%��17.7%��
��100%��17.7%��

����Ŀ����ͼ��ijѧ�����Ƶ�Ԫ�����ڱ���һ���֣�
1H �� | 2He �� | ||||||
3Li � | 4Be �� | 5B �� | 6C ̿ | 7N �� | 8O �� | 9F �� | 10Ne �� |
11Na �� | 12Mg þ | 13Al �� | 14Si �� | 15P �� | 16S �� | 17Cl �� | 18Ar � |
����������Ϣ��֪ʶ�ش�
(1)ԭ�������ֱ�Ϊ8��13����Ԫ����ɵĻ�����Ļ�ѧʽ��____��
(2)��ϸ�Ķ��۲��ϱ���������һ�ֳ���Ԫ�ص�Ԫ��������д������д������ȷ��Ԫ�����ƣ�_____�������ŷŸ�Ԫ�ص�һ�������ﵽ�����У�������____ЧӦ��ʹȫ���ů�����о�����һ����ֹ��ЧӦ��һ����ǿ�Ĵ�ʩ��____��
![]()
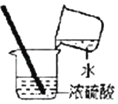
����Ŀ���ס���������ѧ��ȤС��ֱ�����ʵ�����ϵ�����������Һ��ϡ�������ʵ�顣����ͬѧ��һ����ϡ�����������������Һ�У�δ�ܹ۲쵽�����������ǽ���������̽����
ʵ��һ��̽���������ƺ�ϡ�����Ƿ��ܷ�����Ӧ��
��� | ʵ����� | ʵ������ | ʵ����� |
1 | ȡ�μ�ϡ��������Һ��Ʒ���Թ��У��μӷ�̪��Һ | ��Һ���ɫ | ����������ϡ�����Ӧ |
2 | ȡ�μ�ϡ��������Һ��Ʒ���Թ��У�����п�� | ����A | ����������ϡ���ᷢ����Ӧ |
��1������A��_________��
��2�����ڲ�ͬ��ʵ������С�龭������Ϊʵ��_______������1������2�������Ͻ��������ǣ�_______��
����ͬѧ��ϡ���������һ��δ�Ǻ�ƿ��������������Һ��ʵ��ʱ���۲쵽�����ݲ�����һ�����������ǵó������������ѱ��ʡ�
ʵ������ⶨ������������Һ��̼���Ƶ�����������
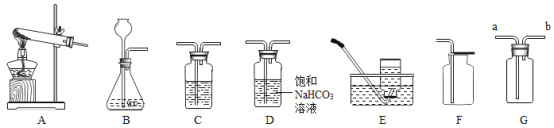
��������ʦ�İ������������ͼװ�ý���ʵ�顣ʵ������Ʒ����ΪM�ˣ������ʵ���Cƿ����������N�ˣ�����M��N���ɼ���ó�����Ʒ��̼���Ƶ�����������

��3������������Һ�ڿ����б��ʵ�ԭ����_________��
��4����û��Bװ�ã�������̼��������������________������ƫ��������ƫС��������������
��5��ʵ���У���A�з�Ӧ��������ͨ����㵪�����ò�����Ŀ����________��