��Ŀ����
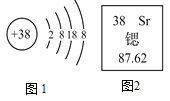
����Ŀ����ͼ����XXX����Ƭ��Ʒ��ǩͼ������ݸñ�ǩ��Ϣ�ش����⡣

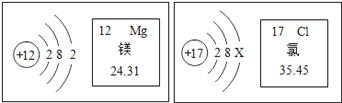
����Ҫ�ɷ�̼����и�Ԫ�ص�ԭ�Ӹ�����___________��
����Ҫ�ɷ�̼��Ƶ���Է�������Ϊ___________��
����Ҫ�ɷ�̼����и�Ԫ�ص���������Ϊ___________��
��ÿƬ�����ٺ���Ԫ�ص�����Ϊ______g��(�������С������λ)
����֪��̼���������ɷֲ���ϡ���ᷴӦ����ÿƬҩƬ������ϡ���ᷴӦ���ٿ��Բ���������̼��������__________g��(�������С������λ)(��Ӧ�ķ���ʽΪ2HCl+CaCO3==CaCl2+H2O+CO2��)
���𰸡� 1:1:3 100 40% 0.50 0.55
����������̼����и�Ԫ�ص�ԭ�Ӹ�����=1��1��3������Ҫ�ɷ�̼��Ƶ���Է�������=40+12+48=100����̼����и�Ԫ�ص���������=![]()
![]() 100%=40%����ÿƬ�����ٺ���Ԫ�ص�����=1.24g
100%=40%����ÿƬ�����ٺ���Ԫ�ص�����=1.24g![]() 40%=0.50g������ÿƬҩƬ������ϡ���ᷴӦ���ٿ��Բ���������̼������Ϊx��
40%=0.50g������ÿƬҩƬ������ϡ���ᷴӦ���ٿ��Բ���������̼������Ϊx��
2HCl+CaCO3==CaCl2+H2O+CO2��
100 44
1.24g x
![]()
x=0.55g��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ