��Ŀ����
�ⶨþ�����ʳ̶ȣ�þ�������ڿ����л����Ϊ��ʽ̼��þ����Ҫ�ⶨһ�������Ϊ��ʽ̼��þ��þ���Ĵ��ȣ����������ϣ���ʽ̼��þ�������ᷴӦ�����Ȼ�þ��ˮ�Ͷ�����̼����ʵ��װ�ã�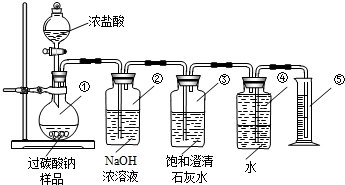
�����������ۡ�
��С��ͬѧ��Ϊ�ⶨ������H2������ȿɲⶨ��Ʒ�Ĵ��ȣ�
��1��װ�âڵ������ǣ�
��2��װ�â۵������ǣ�
��3���ж�þ�������Ƿ���ȫ��Ӧ��ʵ�����������������
��4����ȡ��Ʒ������Ũ�����ַ�Ӧ���ռ������������Ϊ0.448L����֪��ʵ���������������ܶ�Ϊ0.089g/L�������ȡ����Ʒ����Ӧ����
��С��ͬѧ��Ϊ�ⶨ�����е�CO2������������Ӧǰ��װ�âڵ������Ҳ�ɲⶨ��Ʒ�е���þ�Ĵ��ȣ�����������ʵ��װ�ò��CO2��������������Ʒ�Ĵ��ȣ����ƫ�ͣ�����Ϊԭ�������
A��Ũ�����ӷ��������Ȼ������屻װ�â�����
B��������̼�����ݳ�ʱ����ˮ������װ�â���������������
C��װ�â٢��ڿ����ж�����̼��װ�â�����
D��ʵ�����ʱ��ƿ�в���������̼
E�����������������Һ���ݳ��Ǵ�������ˮ������
�����������������ۡ���1������װ�â���Ũ����������Һ�������dz�ȥ������̼���Ȼ���������н��
��2������װ�â��г���ʯ��ˮ����������֤������̼�Ƿ������н��
��3�����ݼ�ʽ̼��þ�������Լ�����þ�����ᷴӦ����ų�������н��
��4����������������Լ��ܶ�������������������������������������þ���������ɣ�
�������Ͽ��Ը������ɶ�����̼��������Ʒ���ȣ�����ʵ��������ͨ��ʵ���ô����Ķ�����̼����������Ϊ��Ӧǰ��װ�âڵ��������л������Ȼ����ˮ����������������������⣮
��2������װ�â��г���ʯ��ˮ����������֤������̼�Ƿ������н��
��3�����ݼ�ʽ̼��þ�������Լ�����þ�����ᷴӦ����ų�������н��
��4����������������Լ��ܶ�������������������������������������þ���������ɣ�
�������Ͽ��Ը������ɶ�����̼��������Ʒ���ȣ�����ʵ��������ͨ��ʵ���ô����Ķ�����̼����������Ϊ��Ӧǰ��װ�âڵ��������л������Ȼ����ˮ����������������������⣮
����⣺��1��װ�â���Ũ����������Һ�������dz�ȥ������̼���Ȼ������壻
��2��װ�â��г���ʯ��ˮ����������֤������̼�Ƿ�����
��3���ж�þ�������Ƿ���ȫ��Ӧ��ʵ����������������ǣ��μ�Ũ����û������ð����
��4������������=0.448L��0.089g/L��0.04g
������0.04g����������Ҫ����þ������Ϊx
Mg+H2SO4�TMgSO4+H2��
24 2
x 0.04g
=
x=0.48g
�������ȡ����Ʒ����Ӧ����0.48g��
��5����ö�����̼�����ƫ��ԭ������У�Ũ�����ӷ����������Ȼ������屻װ�â����գ�������̼�����ݳ�ʱ������ˮ������װ�â��������������գ�װ�â١����ڿ����еĶ�����̼��װ�â����գ�
�ʴ�Ϊ����1����ȥ������̼���Ȼ������壻��2����֤������̼�Ƿ�������3���μ�Ũ����û������ð������4��0.48����ABC��
��2��װ�â��г���ʯ��ˮ����������֤������̼�Ƿ�����
��3���ж�þ�������Ƿ���ȫ��Ӧ��ʵ����������������ǣ��μ�Ũ����û������ð����
��4������������=0.448L��0.089g/L��0.04g
������0.04g����������Ҫ����þ������Ϊx
Mg+H2SO4�TMgSO4+H2��
24 2
x 0.04g
| 24 |
| x |
| 2 |
| 0.04g |
x=0.48g
�������ȡ����Ʒ����Ӧ����0.48g��
��5����ö�����̼�����ƫ��ԭ������У�Ũ�����ӷ����������Ȼ������屻װ�â����գ�������̼�����ݳ�ʱ������ˮ������װ�â��������������գ�װ�â١����ڿ����еĶ�����̼��װ�â����գ�
�ʴ�Ϊ����1����ȥ������̼���Ȼ������壻��2����֤������̼�Ƿ�������3���μ�Ũ����û������ð������4��0.48����ABC��
������������Ҫ������ѧ����ϴ��ƿ�Ľӿ����⡢CO2 ���������ơ��������Ʒ�Ӧ��������;��֪ʶ��ͬʱ������ѧ���������������ۺϷ���������

��ϰ��ϵ�д�
�����Ŀ