��Ŀ����
��֪ij������Ʒ�к���NaCl���ʣ�Ϊ�ⶨ��Ʒ�д��������������������ͼ�е�װ�ý���ʵ�飨��ܰ��ʾ����ʯ�ҵ���Ҫ�ɷ���NaOH��CaO������Ҫʵ�鲽�����£�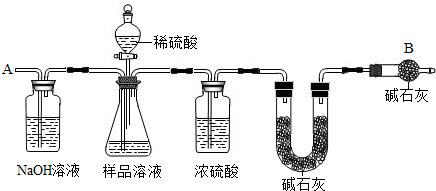
�ٰ�ͼ��װ�����������װ�õ�������
�ڽ�ag��Ʒ������ƿ�У�����������ˮ�ܽ⣬�õ���Ʒ��Һ
�۳���ʢ�м�ʯ�ҵ�U�ܵ��������õ�bg
�ܴӷ�Һ©������ϡ���ᵽ���ٲ�������Ϊֹ
�ݴӵ���A����������һ�����Ŀ���
���ٴγ���ʢ�м�ʯ�ҵ�U�ܵ��������õ�cg
���ظ�����ݺ͢IJ�����ֱ��ʢ�м�ʯ�ҵ�U�ܵ������������䣬Ϊdg
��ش��������⣺
��1������������ƽ������Ʒʱ�������ƽ��ָ������ƫת��˵��
��2��װ����NaOH��Һ��������
��3��װ����Ũ�����������
��4���������Һ©���е�ϡ���ỻ��Ũ����ͬ�����ᣬ�ⶨ�Ľ����
��5������ݵ�Ŀ����
��6������Ʒ�д�������������ļ���ʽΪ��
��������1������������ƽ��ʹ�÷������������ֵ�������з������жϣ���̼������ӵ��������ӶԽ��з���
��2�����ݿ�������ɼ��������Ƶ����ʣ�����ͨ������������Һ�п�����ɵĸı䣬�ж�����������Һ�����ã�
��3����Ũ��������Է���ʵ���е���;��
��4����Ũ��������ԣ���������Ũ�����Բⶨ�����ɵ�Ӱ�죻
��5����Ӧ���װ���ڻ��в���Ķ�����̼���ܽ���U�ܣ���Ӱ��ⶨ�����
��6������������������=
��100%��������������֪������Ҫ��������������U��ǰ�����ӵ������Ƿ�Ӧ���ɶ�����̼�����������ݶ�����̼����������������������
��2�����ݿ�������ɼ��������Ƶ����ʣ�����ͨ������������Һ�п�����ɵĸı䣬�ж�����������Һ�����ã�
��3����Ũ��������Է���ʵ���е���;��
��4����Ũ��������ԣ���������Ũ�����Բⶨ�����ɵ�Ӱ�죻
��5����Ӧ���װ���ڻ��в���Ķ�����̼���ܽ���U�ܣ���Ӱ��ⶨ�����
��6������������������=
| ��������� |
| ���������� |
����⣺��1����ƽ��ָ������ƫת�����ݡ��������롱��˵����Ʒ�������㣻
��2�������еĶ�����̼�ܱ�NaOH��Һ���գ�ͨ��NaOH��Һ��Ŀ����в��ٺ��ж�����̼�������˿����ж�����̼�Բⶨ�����Ӱ�죻
��3��Ũ���������ˮ�ԣ��������ͨ��Ũ����ʱ���������������е�ˮ������ˮ�Բⶨ�����Ӱ�죻
��4����ϡ����Ũ����ͬ��������к�ǿ�Ļӷ��ԣ��ӷ�������HCl�����ܱ���ʯ�������գ���˻�ʹ��ʯ����������ֵƫ��ʹ�ⶨ���ƫ�ߣ�
��5���ӵ���A����������һ�����Ŀ�������������������������Һ�Ĵ����������������ɰѲ����ڷ�Ӧװ���еĶ�����̼ȫ���ų���ʹ�òⶨ�������ȷ��
��6���贿�������Ϊx
Na2CO3+H2SO4�TNa2SO4+CO2��+H2O
106 44
x d-b
=
x=
����Ʒ�д������������=
��100%
�ʴ�Ϊ��
��1����Ʒ�������㣻
��2����ȥ�����еĶ�����̼��
��3����ȥ������̼�е�ˮ������
��4��ƫ�ߣ�
��5��ʹ���ɵĶ�����̼���御���ܱ�U���еļ�ʯ�����գ�
��6��
��100%��
��2�������еĶ�����̼�ܱ�NaOH��Һ���գ�ͨ��NaOH��Һ��Ŀ����в��ٺ��ж�����̼�������˿����ж�����̼�Բⶨ�����Ӱ�죻
��3��Ũ���������ˮ�ԣ��������ͨ��Ũ����ʱ���������������е�ˮ������ˮ�Բⶨ�����Ӱ�죻
��4����ϡ����Ũ����ͬ��������к�ǿ�Ļӷ��ԣ��ӷ�������HCl�����ܱ���ʯ�������գ���˻�ʹ��ʯ����������ֵƫ��ʹ�ⶨ���ƫ�ߣ�
��5���ӵ���A����������һ�����Ŀ�������������������������Һ�Ĵ����������������ɰѲ����ڷ�Ӧװ���еĶ�����̼ȫ���ų���ʹ�òⶨ�������ȷ��
��6���贿�������Ϊx
Na2CO3+H2SO4�TNa2SO4+CO2��+H2O
106 44
x d-b
| 106 |
| x |
| 44 |
| d-b |
| 106(d-b) |
| 44 |
����Ʒ�д������������=
| 106(d-b) |
| 44a |
�ʴ�Ϊ��
��1����Ʒ�������㣻
��2����ȥ�����еĶ�����̼��
��3����ȥ������̼�е�ˮ������
��4��ƫ�ߣ�
��5��ʹ���ɵĶ�����̼���御���ܱ�U���еļ�ʯ�����գ�
��6��
| 106(d-b) |
| 44a |
��������ʵ���װ������˳��dz����ܣ���ǰ�������������Һ��Ϊ�˷�ֹ�����еĶ�����̼����װ�ã������ļ�ʯ��Ҳ��Ϊ�˷�ֹ�����еĶ�����̼����װ�ã�

��ϰ��ϵ�д�
�����Ŀ


