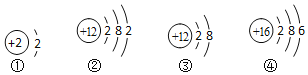��Ŀ����
����Ŀ��ˮ������֮Դ��Ҳ������������Դ������ѧ���Ļ�ѧ֪ʶ�ش���������
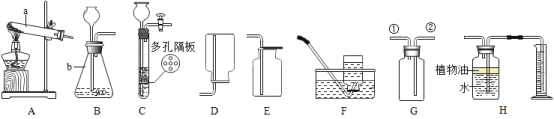
��1�������ʵķ���Ƕȷ�����ˮ������________������ĸ����
a ���� b ������ c ������ d ������
��2������ȥˮ�в��������ʣ�����еIJ�����________��
��3��Ӳˮ�к��н϶�����Ըơ�þ�����Ӳˮ���������������������鷳�������п���_______����������ˮ��Ӳ�ȡ�
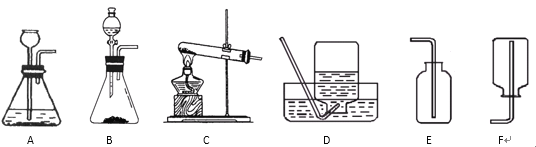
��4����������ͼ�ش��������⣺
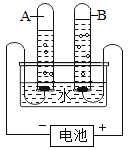
�Թ� A �е������������_______����ʵ��֤��ˮ����________��ɵġ�
��5��ͨ������Ȼ���е�ˮͶ�Ÿ������ƣ���ѧʽΪ Na2FeO4��ɱ�������� ������������Ԫ�صĻ��ϼ�Ϊ________��
��6���ҹ��йز��Ź涨������������û�������ˮ����<0.3mg��L-1����ͭ<1.0mg��L -1�����е�������ͭ��ָ����________������ȷѡ��ǰ����ĸ����
A ԭ�� B ���� C Ԫ��
���𰸡�A ���� ��� ���� ��Ԫ�غ���Ԫ�� +6 C
��������
��1��ˮ��ˮ���ӹ��ɣ������Ǵ����ˮ����������Ԫ�غ���Ԫ����ɵģ������ǻ����������һ������Ԫ�أ��������������A���������⡣�ʴ�Ϊ��A��
��2����ȥ���������ʣ������ǹ��ˡ��ʴ�Ϊ�����ˣ�
��3�������п�����з�������ˮӲ�ȣ�ʵ���ҳ������ʴ�Ϊ����У�
��4����ͼ��֪ A �Ӹ�����������Ϊ������ˮͨ��ֽ������������������ˮ������Ԫ�غ���Ԫ����ɵġ��ʴ�Ϊ����������Ԫ�غ���Ԫ�أ�
��5������Ԫ�ػ��ϼ�ΪX������Ԫ�ػ��ϼ�Ϊ+1����Ԫ��-2�����ԣ�+1����2+��-2����4+X=0������ X=+6���ʴ�Ϊ��+6��
��6������ˮ�еĺ���ͭ������ָԪ�ء��ʴ�Ϊ��C��