��Ŀ����
Ϊ�ⶨij��������Һ���������ĺ�����ȡ20g ��Һ���ձ��У�����5.5g���ᣨ������ʹ��Һ�е���������ȫ��Ӧ���������İ�ɫ�������ˡ�ϴ�ӡ��������Ϊ2.87g����
��1������20g��Һ��AgNO3��������
��2����ʵ������У�ͨ����ͨ����ȡҺ��������ȡ��һ������Һ�壮�������������ʵ�������µ��ܶ�Ϊ1.1g/mL�����ڱ�ʵ�������õ�5.5g ���������Ƕ��ٺ�����
�⣺��1������Һ��AgNO3������Ϊx
AgNO3+HCl=AgCl��+HNO3
170 143.5
x 2.87g
 x=3.4g
x=3.4g
��2�����õ�5.5g ��������=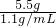 =5mL
=5mL
����Һ��AgNO3������Ϊ3.4g������������5mL��
��������1�������������ᷢ�����ֽⷴӦ�������Ȼ�����ɫ�����������ƣ����ݷ�Ӧ�Ļ�ѧ����ʽ���ɵ�֪�����Ȼ�������������������ϵ�����������ʵ������ɳ����Ȼ����������ɼ���μӷ�Ӧ��������������
��2��ʹ����Һ�����������ϵ��m=��V����������������ܶȿɼ�������������
���������ݻ�ѧ����ʽ�ɱ�ʾ��Ӧ�и����ʵ�������ϵ�����÷�Ӧ�����ʵ�������ϵ�����ɷ�Ӧ����֪���ʵ��������㷴Ӧ���������ʵ�������
AgNO3+HCl=AgCl��+HNO3
170 143.5
x 2.87g
 x=3.4g
x=3.4g��2�����õ�5.5g ��������=
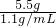 =5mL
=5mL����Һ��AgNO3������Ϊ3.4g������������5mL��
��������1�������������ᷢ�����ֽⷴӦ�������Ȼ�����ɫ�����������ƣ����ݷ�Ӧ�Ļ�ѧ����ʽ���ɵ�֪�����Ȼ�������������������ϵ�����������ʵ������ɳ����Ȼ����������ɼ���μӷ�Ӧ��������������
��2��ʹ����Һ�����������ϵ��m=��V����������������ܶȿɼ�������������
���������ݻ�ѧ����ʽ�ɱ�ʾ��Ӧ�и����ʵ�������ϵ�����÷�Ӧ�����ʵ�������ϵ�����ɷ�Ӧ����֪���ʵ��������㷴Ӧ���������ʵ�������

��ϰ��ϵ�д�
�����Ŀ