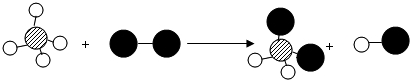
��Ŀ����
�Ȼ�������Ҫ�ĵ�ζƷ������������ȱ�ٵ�ζ������������ʾ��ͼ�ֱ��ʾ��ͬ�Ļ�ѧ��Ӧ�����������ж����Ȼ��ƣ�
��1��ͼ1�ǽ�������������Ӧ�����Ȼ��Ƶ���ʾ��ͼ����ͼ1��֪��Ԫ�صĻ�ѧ������______������ĸ��ţ������еĹ�ϵ��A������������B���ڲ������C�����Ӳ���
��2��ͼ2������NaOH��Һ�����ᷴӦ����ʵ�ʣ�ͼ��A��B��C��Ӧ����Ļ�ѧʽ�����ӷ�������Ϊ______���÷�Ӧ�Ļ�ѧ����ʽΪ______��
��3��ͼ3��ʾ�������������������������______���ѧʽ�����÷�Ӧ�Ļ�ѧ����ʽΪ______��

��1��ͼ1�ǽ�������������Ӧ�����Ȼ��Ƶ���ʾ��ͼ����ͼ1��֪��Ԫ�صĻ�ѧ������______������ĸ��ţ������еĹ�ϵ��A������������B���ڲ������C�����Ӳ���
��2��ͼ2������NaOH��Һ�����ᷴӦ����ʵ�ʣ�ͼ��A��B��C��Ӧ����Ļ�ѧʽ�����ӷ�������Ϊ______���÷�Ӧ�Ļ�ѧ����ʽΪ______��
��3��ͼ3��ʾ�������������������������______���ѧʽ�����÷�Ӧ�Ļ�ѧ����ʽΪ______��
��1����ͼ��֪����������ѧ�仯ʱ��Ԫ��ԭ�ӵ����������������˸ı䣬��Ԫ�صĻ�ѧ���������������������е���ϵ��
��2����������������ķ�Ӧ�������ֻ�����������ɷ֣����������µĻ�����ķ�Ӧ�����ڸ��ֽⷴӦ���˷�Ӧ��ʵ�������е������Ӻͼ��е����������ӽ��������ˮ���ӣ��������Ƶ����A�����������ӣ�����������B�������ӣ����ɵ�C��ˮ���ӣ���A��B��C�����ӷ��ŷֱ���OH-��H+��H2O���÷�Ӧ�ķ���ʽΪNaOH+HCl=NaCl+H2O��
��3��ͼ3�� ��������Ԫ������Һ�����Ԫ�صĻ��������������仯ѧʽΪClO2���÷�Ӧ�Ļ�ѧ����ʽΪCl2+2NaClO2�T2ClO2+2NaCl��
��������Ԫ������Һ�����Ԫ�صĻ��������������仯ѧʽΪClO2���÷�Ӧ�Ļ�ѧ����ʽΪCl2+2NaClO2�T2ClO2+2NaCl��
�ʴ�Ϊ����1��A����2��OH-��H+��H2O��NaOH+HCl=NaCl+H2O����3��ClO2��Cl2+2NaClO2�T2ClO2+2NaCl��
��2����������������ķ�Ӧ�������ֻ�����������ɷ֣����������µĻ�����ķ�Ӧ�����ڸ��ֽⷴӦ���˷�Ӧ��ʵ�������е������Ӻͼ��е����������ӽ��������ˮ���ӣ��������Ƶ����A�����������ӣ�����������B�������ӣ����ɵ�C��ˮ���ӣ���A��B��C�����ӷ��ŷֱ���OH-��H+��H2O���÷�Ӧ�ķ���ʽΪNaOH+HCl=NaCl+H2O��
��3��ͼ3��
 ��������Ԫ������Һ�����Ԫ�صĻ��������������仯ѧʽΪClO2���÷�Ӧ�Ļ�ѧ����ʽΪCl2+2NaClO2�T2ClO2+2NaCl��
��������Ԫ������Һ�����Ԫ�صĻ��������������仯ѧʽΪClO2���÷�Ӧ�Ļ�ѧ����ʽΪCl2+2NaClO2�T2ClO2+2NaCl���ʴ�Ϊ����1��A����2��OH-��H+��H2O��NaOH+HCl=NaCl+H2O����3��ClO2��Cl2+2NaClO2�T2ClO2+2NaCl��

��ϰ��ϵ�д�
�����Ŀ

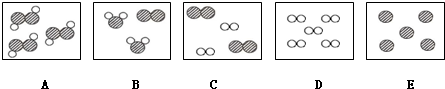
 ����ʾ��ԭ�ӣ��á�
����ʾ��ԭ�ӣ��á� ����ʾ��ԭ�ӣ��á�
����ʾ��ԭ�ӣ��á� ����ʾ��ԭ�ӣ�������Ӧ���̿�����ͼ��ʾΪ��
����ʾ��ԭ�ӣ�������Ӧ���̿�����ͼ��ʾΪ��
 ����
���� ���ֱ��ʾ����Ԫ�ص�ԭ�ӣ���ϸ�۲�ͼ���ж�����˵����ȷ���ǣ�������
���ֱ��ʾ����Ԫ�ص�ԭ�ӣ���ϸ�۲�ͼ���ж�����˵����ȷ���ǣ�������