��Ŀ����
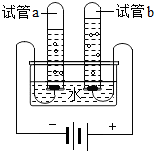 ˮ������������Ȼ��Դ��������ѧ��ѧ֪ʶ�ش�
ˮ������������Ȼ��Դ��������ѧ��ѧ֪ʶ�ش���1��С��������ͼ��ʾ��װ��̽��ˮ����ɣ�д���÷�Ӧ�Ļ�ѧʽ����ʽ
2H2O
2H2��+O2��
| ||
2H2O
2H2��+O2��
��ͨ��һ��ʱ����Թ�a�ռ�����������
| ||
����
����
�������븺������������������1��2
1��2
��Ϊ����ǿˮ�ĵ�����
��ǿˮ�ĵ�����
����Ҫ��ˮ�м���ϡ���������������Һ����2�������϶�һ�š�����̫�յĻ����������Ϊ����ʹ�õ�ȼ����Һ�⡢��ȼ����Һ������Һ��ȼ�յĻ�ѧʽ����ʽΪ
2H2+O2
2H2O
| ||
2H2+O2
2H2O
��
| ||
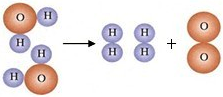
��3��Ϊ��̽����ˮ�ľ��������̣�ijʵ��С��ӻ��Ǻ���ȡ��ˮ�����۲쵽ˮ�����ǣ��ʻ�ɫ������ζ���й���С�������ֶ�ˮ���������´������裺
�ټ������̿����ˮ�е���ɫ����ζ��
����ˮ���м������������ܽ⣬����һ��ʱ�����
����
����
����������ƣ�����ȥ��ˮ���еĹ���С��������������ò���������Һ�Ծɻ��ǣ������һ�����ԭ���������ֽ���𣬹���ʱҺ�������ֽ�ı��ص�
��ֽ���𣬹���ʱҺ�������ֽ�ı��ص�
����4������ˮ���ö�����������ɱ�����������ȵĻ�ѧʽΪ
ClO2
ClO2
��������Ԫ�صĻ��ϼ�Ϊ+4
+4
����5����ˮ�к��н϶�ĸơ�þ���ӣ�Ϊ����Ӳ�ȣ��ճ�������ʹ��Ӳˮ����������鷳����ͥ�����г���������ˮӲ�ȵķ�����
���
���
����������1�����ݵ��ˮʵ�������ͽ��ۼ�ע�����������ش�
��2����������ȼ�յķ�Ӧд����Ӧ�Ļ�ѧʽ����ʽ��
��3���ڸ��ݹ��˵�ԭ��������ע����������ش�
��4����������д����ѧʽ�����ݻ��ϼ�ԭ�����Ԫ�صĻ��ϼۣ�
��5�����ݼ�ͥ������Ӳˮ�������õķ��������ش�
��2����������ȼ�յķ�Ӧд����Ӧ�Ļ�ѧʽ����ʽ��
��3���ڸ��ݹ��˵�ԭ��������ע����������ش�
��4����������д����ѧʽ�����ݻ��ϼ�ԭ�����Ԫ�صĻ��ϼۣ�
��5�����ݼ�ͥ������Ӳˮ�������õķ��������ش�
����⣺��1�����ˮ��������������������Ӧ�Ļ�ѧʽ����ʽ�ǣ�2H2O
2H2��+O2�� ��װ�ÿ�֪��ͨ��һ��ʱ����Թ�a�ռ����������ǵ�Դ�ĸ������������壬�϶��������������븺������������������1��2��Ϊ����ǿˮ�ĵ����ԣ���Ҫ��ˮ�м���ϡ���������������Һ��
��2��Һ��ȼ��������ˮ����Ӧ�Ļ�ѧʽ����ʽΪ��2H2+O2
2H2O��
��3������ˮ���м������������ܽ⣬����һ��ʱ����й��ˣ���ȥ��ˮ���еĹ���С��������������ò���������Һ�Ծɻ��ǣ������һ�����ԭ������ǣ���ֽ���𣬹�����Һ�������ֽ�ı��صȣ�
��4������ˮ���ö�����������ɱ�����������ȵĻ�ѧʽΪ��ClO2����������-2�ۣ�������Ԫ�صĻ��ϼ�Ϊ+4��
��5����ˮ�к��н϶�ĸơ�þ���ӣ�Ϊ����Ӳ�ȣ�ͨ������ܽ��ơ�þ���ӵĻ����ﺬ�������ԣ���ͥ�����г�����еķ���������ˮ��Ӳ�ȣ�
�ʴ�Ϊ����1��2H2O
2H2��+O2����������1��2����ǿˮ�ĵ����ԣ���2��2H2+O2
2H2O����3�����ˣ���ֽ���𡢹���ʱҺ�������ֽ�ı��صȣ���4��ClO2��+4����5����У�
| ||
��2��Һ��ȼ��������ˮ����Ӧ�Ļ�ѧʽ����ʽΪ��2H2+O2
| ||
��3������ˮ���м������������ܽ⣬����һ��ʱ����й��ˣ���ȥ��ˮ���еĹ���С��������������ò���������Һ�Ծɻ��ǣ������һ�����ԭ������ǣ���ֽ���𣬹�����Һ�������ֽ�ı��صȣ�
��4������ˮ���ö�����������ɱ�����������ȵĻ�ѧʽΪ��ClO2����������-2�ۣ�������Ԫ�صĻ��ϼ�Ϊ+4��
��5����ˮ�к��н϶�ĸơ�þ���ӣ�Ϊ����Ӳ�ȣ�ͨ������ܽ��ơ�þ���ӵĻ����ﺬ�������ԣ���ͥ�����г�����еķ���������ˮ��Ӳ�ȣ�
�ʴ�Ϊ����1��2H2O
| ||
| ||
������������Ҫ������ˮ�ĵ�⡢���ɼ�������֪ʶ��ˮ������������Ȼ��Դ��Ӧ��ǿˮ��֪ʶ��ѧϰ������ˮ��֪ʶ��

��ϰ��ϵ�д�
 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
�����Ŀ

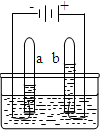
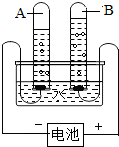 ˮ������������Ȼ��Դ����ͼ�ǵ��ˮ��ʵ��װ�ã��ش��������⣺
ˮ������������Ȼ��Դ����ͼ�ǵ��ˮ��ʵ��װ�ã��ش��������⣺ ��2010?�Թ���ˮ������������Ȼ��Դ����ͼ�ǵ��ˮ��ʵ��װ�ã��ش��������⣺
��2010?�Թ���ˮ������������Ȼ��Դ����ͼ�ǵ��ˮ��ʵ��װ�ã��ش��������⣺ ˮ������������Ȼ��Դ��С��������ͼ��ʾ��װ��̽��ˮ����ɣ�ͨ��һ��ʱ����Թ�A���Թ�B���ռ������������֮��ԼΪ
ˮ������������Ȼ��Դ��С��������ͼ��ʾ��װ��̽��ˮ����ɣ�ͨ��һ��ʱ����Թ�A���Թ�B���ռ������������֮��ԼΪ