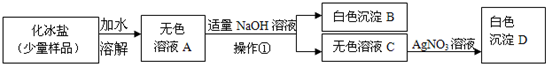
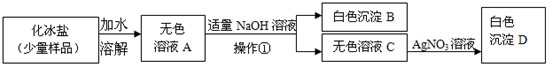
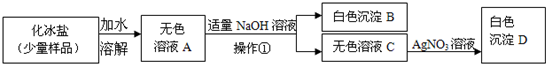
��Ŀ����
��2008?��Ǩ���ҹ���ѧ�Һ�°�����һ�������Ĵ�����������----�������Ƽ������1���������Ƽ�����������û�ѧ����ʽ�ɼ�Ҫ��ʾΪ��
��NH3+CO2+H2O=NH4HCO3
��NH4HCO3+NaCl=NaHCO3��+NH4Cl
��2NaHCO3=Na2CO3+H2O+CO2��
�ù�����û���漰�Ļ�����Ӧ������______
��2���ڢڲ��м������ĥϸʳ�ηۣ�ʳ��ĥϸ��Ŀ����______��
�Ӹò���Ӧ�����Ի��______��
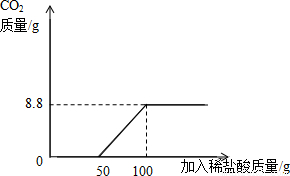
��3�����������ƵõĴ����Ʒ�г������������Ȼ��ƣ�ij�о���ѧϰС��Ը��������Ĵ����Ʒ���м�⣮ȡ22�˸���Ʒ���ձ��У���ˮ�����ܽ⣬Ȼ����μ���������������Ϊ14.6%��ϡ���Ტ���������ȷ����ķ�Ӧ�ǣ�Na2CO3+HCl=NaHCO3+NaCl��Ȼ�����ķ�Ӧ�ǣ�NaHCO3+HCl=NaCl+H2O+CO2�����������������ձ�����Һ���������ϡ���������Ĺ�ϵ��ͼ����ʾ��

���������ͼ���ṩ����Ϣ����ͼ�ҵ�����ϵ�л����������в���CO2��������μ����������ı仯���ߣ�������������̼��������Ӧ��ֵ��
�ڼ������Ʒ�д�����������������������ðٷ�����ʾ��������С�����һλ��
���𰸡�����������̼���Ƶ���ȡ���̽��з�������1��������Ӧ�Ļ�ѧ����ʽ����֪����Ӧ�����ͣ���2����������Ĵ�СӰ�췴Ӧ���ٶȣ�������Ϊ�Ȼ�泥����ڵ��ʣ���3���ټ������ʼ����������̼�ĵ�Ͳ��ٲ���������̼�ĵ㼴�ɻ�ñ仯�����ߣ���Ҫ����̼���Ƶ�������������Ҫ���̼���Ƶ�������
����⣺��1�������ڻ��Ϸ�Ӧ�������ڸ��ֽⷴӦ�������ڷֽⷴӦ�����漰�û���Ӧ�����Ա����Ϊ���û���Ӧ��
��2����������Ĵ�СӰ�췴Ӧ���ٶȣ�������Ϊ�Ȼ�泥����ڵ��ʣ����Ա����Ϊ���ӿ컯ѧ��Ӧ���ʻ�ӿ�ʳ���ܽ⣬����
��3���پ�ͼ���Կ������������������Ϊ50gʱ��ʼ����������̼���������������Ϊ100gʱ�������ɶ�����̼�������������̼������Ϊx������
NaHCO3+HCl=NaCl+H2O+CO2����
36.5 44
50g×14.6% x
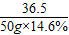 =
=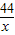
x=8.8g
���Ա����Ϊ��
��4������������Կ�������̼���Ʒ�Ӧ�����Ṳ��100g����̼���Ƶ�����Ϊy������
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73
y 100g×14.6%
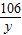 =
=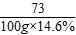
y=21.2g
���Ը���Ʒ�д������������Ϊ��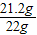 ×100%=96.4%
×100%=96.4%
���������⿼����̼���Ƶ���ȡ�Լ��йصļ��㣬��ɴ��⣬�����������е�֪ʶ�������ṩ����Ϣ���У�
����⣺��1�������ڻ��Ϸ�Ӧ�������ڸ��ֽⷴӦ�������ڷֽⷴӦ�����漰�û���Ӧ�����Ա����Ϊ���û���Ӧ��
��2����������Ĵ�СӰ�췴Ӧ���ٶȣ�������Ϊ�Ȼ�泥����ڵ��ʣ����Ա����Ϊ���ӿ컯ѧ��Ӧ���ʻ�ӿ�ʳ���ܽ⣬����
��3���پ�ͼ���Կ������������������Ϊ50gʱ��ʼ����������̼���������������Ϊ100gʱ�������ɶ�����̼�������������̼������Ϊx������
NaHCO3+HCl=NaCl+H2O+CO2����
36.5 44
50g×14.6% x
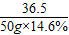 =
=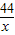
x=8.8g
���Ա����Ϊ��

��4������������Կ�������̼���Ʒ�Ӧ�����Ṳ��100g����̼���Ƶ�����Ϊy������
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73
y 100g×14.6%
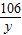 =
=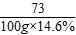
y=21.2g
���Ը���Ʒ�д������������Ϊ��
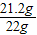 ×100%=96.4%
×100%=96.4%���������⿼����̼���Ƶ���ȡ�Լ��йصļ��㣬��ɴ��⣬�����������е�֪ʶ�������ṩ����Ϣ���У�

��ϰ��ϵ�д�
 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
�����Ŀ