��Ŀ����
д�����л�ѧ�仯�Ļ�ѧ����ʽ������й�����
(1)ľ̿��������ȼ�գ�
________________________________����������________________���顣
(2)������̼ͨ�������ɫʯ����Һ��ˮ�У�
________________________________���÷�Ӧ����Ϊ________________��
(3) ʵ�����ù�������Ͷ���������ȡ������_______________________���÷�Ӧǰ��������̵�_______________________��_______________________���䣬�Ƿ�Ӧ��_______________________
(4) ������ϡ���ᷴӦ��_______________________��
������Ӧ����_______________________����Ӧ����Һ����ɫ��Ϊ_______________________��
(5)����ʯ��ˮ���Լ�ƿ�ڱڳ����γ�һ���ɫ���壺___________________________��ϴȥ�ð�ɫ������Ҫ�õ��Լ���________________�������ƣ�
(1)ľ̿��������ȼ�գ�
________________________________����������________________���顣
(2)������̼ͨ�������ɫʯ����Һ��ˮ�У�
________________________________���÷�Ӧ����Ϊ________________��
(3) ʵ�����ù�������Ͷ���������ȡ������_______________________���÷�Ӧǰ��������̵�_______________________��_______________________���䣬�Ƿ�Ӧ��_______________________
(4) ������ϡ���ᷴӦ��_______________________��
������Ӧ����_______________________����Ӧ����Һ����ɫ��Ϊ_______________________��
(5)����ʯ��ˮ���Լ�ƿ�ڱڳ����γ�һ���ɫ���壺___________________________��ϴȥ�ð�ɫ������Ҫ�õ��Լ���________________�������ƣ�
��1��C��O2 CO2 ����ʯ��ˮ
��2��CO2��H2O H2CO3 ���
H2CO3 ���
��3��2H2O2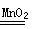 2H2O+O2�� ���� ��ѧ���� ����
2H2O+O2�� ���� ��ѧ���� ����
��4��Fe��2HCl FeCl2��H2�� �û� dz��ɫ
FeCl2��H2�� �û� dz��ɫ
��5��CO2��Ca(OH)2 CaCO3 ����H2O ϡ����
CaCO3 ����H2O ϡ����
��2��CO2��H2O
 H2CO3 ���
H2CO3 �����3��2H2O2
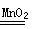 2H2O+O2�� ���� ��ѧ���� ����
2H2O+O2�� ���� ��ѧ���� ������4��Fe��2HCl
 FeCl2��H2�� �û� dz��ɫ
FeCl2��H2�� �û� dz��ɫ��5��CO2��Ca(OH)2
 CaCO3 ����H2O ϡ����
CaCO3 ����H2O ϡ��������1������̼�����������Ļ�ѧ���ʿɵã�ľ̿��������ȼ�յĻ�ѧ����ʽΪ����C��O2 = CO2�����ݶ�����̼�ļ��鷽����֪����ȼ�ղ�����ó����ʯ��ˮ���飮
��2�����ݶ�����̼�Ļ�ѧ���ʿɵã�������̼ͨ�������ɫʯ����Һ��ˮ�з����Ļ�ѧ����ʽΪ��CO2+H2O=H2CO3���÷�Ӧ����Ϊ��ɫʯ����Һ��ɺ�ɫ��
��3������������ʵ������ȡ��Ӧԭ���ʹ����ĸ���ɵã�ʵ�����ù�������Ͷ���������ȡ�����Ļ�ѧ����ʽΪ��2H2O2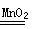 2H2O+O2�����÷�Ӧǰ��������̵������ͻ�ѧ���ʲ��䣬�Ƿ�Ӧ�Ĵ�����
2H2O+O2�����÷�Ӧǰ��������̵������ͻ�ѧ���ʲ��䣬�Ƿ�Ӧ�Ĵ�����
��4������������ϡ���ᣩ�Ļ�ѧ���ʣ��Լ��û���Ӧ�ĸ�����ص�ɵã�������ϡ���ᷴӦ�Ļ�ѧ����ʽΪ��Fe+2HCl=FeCl2+H2����������Ӧ�����û���Ӧ����Ӧ����Һ����ɫ��Ϊdz��ɫ��
��5�����ݶ�����̼�����������ƣ��Ļ�ѧ���ʣ��Լ�̼��ƣ���ϡ���ᣩ�Ļ�ѧ���ʿɵã�����ʯ��ˮ���Լ�ƿ�ڱڳ����γ�һ���ɫ���壬�÷�Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+CO2=CaCO3��+H2O��ϴȥ�ð�ɫ������Ҫ�õ��Լ���ϡ���ᣮ
��2�����ݶ�����̼�Ļ�ѧ���ʿɵã�������̼ͨ�������ɫʯ����Һ��ˮ�з����Ļ�ѧ����ʽΪ��CO2+H2O=H2CO3���÷�Ӧ����Ϊ��ɫʯ����Һ��ɺ�ɫ��
��3������������ʵ������ȡ��Ӧԭ���ʹ����ĸ���ɵã�ʵ�����ù�������Ͷ���������ȡ�����Ļ�ѧ����ʽΪ��2H2O2
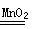 2H2O+O2�����÷�Ӧǰ��������̵������ͻ�ѧ���ʲ��䣬�Ƿ�Ӧ�Ĵ�����
2H2O+O2�����÷�Ӧǰ��������̵������ͻ�ѧ���ʲ��䣬�Ƿ�Ӧ�Ĵ�������4������������ϡ���ᣩ�Ļ�ѧ���ʣ��Լ��û���Ӧ�ĸ�����ص�ɵã�������ϡ���ᷴӦ�Ļ�ѧ����ʽΪ��Fe+2HCl=FeCl2+H2����������Ӧ�����û���Ӧ����Ӧ����Һ����ɫ��Ϊdz��ɫ��
��5�����ݶ�����̼�����������ƣ��Ļ�ѧ���ʣ��Լ�̼��ƣ���ϡ���ᣩ�Ļ�ѧ���ʿɵã�����ʯ��ˮ���Լ�ƿ�ڱڳ����γ�һ���ɫ���壬�÷�Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+CO2=CaCO3��+H2O��ϴȥ�ð�ɫ������Ҫ�õ��Լ���ϡ���ᣮ

��ϰ��ϵ�д�
 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д�
�����Ŀ