��Ŀ����
�±�ΪԪ�����ڱ���ijһ����Ԫ�ص�ԭ�ӽṹʾ��ͼ��
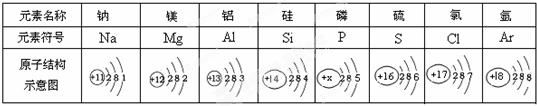
��ش��������⣺
��1��������ԭ�ӵĺ˵����x=
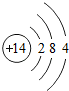
��2�����о�������ȶ��ṹ��Ԫ����
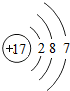
��3���ڻ�ѧ��Ӧ�У�þԭ������ʧȥ�����γ�
˵��Ԫ�صĻ�ѧ������ԭ�ӵ�
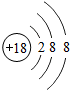
��4����Ԫ����ؿ��к�������Ԫ���γɵĻ����ﻯѧʽΪ
��5������Ԫ�������ڱ��д���ͬһ���ڵ�ԭ����
��6�����в�ͬ��Ԫ����ʵ�������
A�����ԭ��������ͬ B����������ͬ C����������ͬ��

��ش��������⣺
��1��������ԭ�ӵĺ˵����x=
15
15
����2�����о�������ȶ��ṹ��Ԫ����
Ar
Ar
��������ţ���3���ڻ�ѧ��Ӧ�У�þԭ������ʧȥ�����γ�
Mg2+
Mg2+
��������ţ�˵��Ԫ�صĻ�ѧ������ԭ�ӵ�
����������
����������
��ϵ���У���4����Ԫ����ؿ��к�������Ԫ���γɵĻ����ﻯѧʽΪ
Al2O3
Al2O3
����5������Ԫ�������ڱ��д���ͬһ���ڵ�ԭ����
����ԭ�ӵĺ�����Ӳ�����ͬ
����ԭ�ӵĺ�����Ӳ�����ͬ
����6�����в�ͬ��Ԫ����ʵ�������
B
B
������ţ���A�����ԭ��������ͬ B����������ͬ C����������ͬ��
��������1��������ԭ�ӵĽṹʾ��ͼ���н��
��2������Ԫ�صĻ�ѧ���ʸ�����ԭ�ӵ�����������Ŀ��ϵ�dz����н��н��
��3�����ݻ����ﻯѧʽ����д�������н��
��4�����ݽṹʾ��ͼ�۲�ݱ���ɣ�
��2������Ԫ�صĻ�ѧ���ʸ�����ԭ�ӵ�����������Ŀ��ϵ�dz����н��н��
��3�����ݻ����ﻯѧʽ����д�������н��
��4�����ݽṹʾ��ͼ�۲�ݱ���ɣ�
����⣺��1��������ԭ�ӵĽṹʾ��ͼ��֪����ԭ�Ӻ�����15�����ӣ���ԭ�ӵĺ˵����ҲΪ15��
��2��Ԫ�صĻ�ѧ���ʸ�����ԭ�ӵ�����������Ŀ��ϵ�dz����У�������������Ϊ8�����������ȶ��ṹ�����Ծ�������ȶ��ṹ��Ԫ�����Ԫ�أ�
��3���ڻ�ѧ��Ӧ�У�þԭ������ʧȥ2�����ӳ�Ϊþ���ӣ�����ΪMg2+��Ԫ�صĻ�ѧ������ԭ�ӵ� �����������йأ���������������4����ʧȥ���ӣ�
��4���ؿ��к�������Ԫ������Ԫ�أ���Ԫ������Ԫ���γɵĻ����ﻯѧʽΪAl2O3��
��5����ԭ�ӽṹʾ��ͼ���Կ���������ͬһ���ڵ�ԭ�ӣ�������Ӳ�����ͬ����
��6��Ԫ���Ǿ�����ͬ�˵������һ��ԭ�ӵ��ܳƣ���������������Ԫ�����࣮
�ʴ�Ϊ��
��1��15��
��2��Ar��
��3��Mg2+��������������
��4��Al2O3��
��5������ԭ�ӵĺ�����Ӳ�����ͬ��
��6��B��
��2��Ԫ�صĻ�ѧ���ʸ�����ԭ�ӵ�����������Ŀ��ϵ�dz����У�������������Ϊ8�����������ȶ��ṹ�����Ծ�������ȶ��ṹ��Ԫ�����Ԫ�أ�
��3���ڻ�ѧ��Ӧ�У�þԭ������ʧȥ2�����ӳ�Ϊþ���ӣ�����ΪMg2+��Ԫ�صĻ�ѧ������ԭ�ӵ� �����������йأ���������������4����ʧȥ���ӣ�
��4���ؿ��к�������Ԫ������Ԫ�أ���Ԫ������Ԫ���γɵĻ����ﻯѧʽΪAl2O3��
��5����ԭ�ӽṹʾ��ͼ���Կ���������ͬһ���ڵ�ԭ�ӣ�������Ӳ�����ͬ����
��6��Ԫ���Ǿ�����ͬ�˵������һ��ԭ�ӵ��ܳƣ���������������Ԫ�����࣮
�ʴ�Ϊ��
��1��15��
��2��Ar��
��3��Mg2+��������������
��4��Al2O3��
��5������ԭ�ӵĺ�����Ӳ�����ͬ��
��6��B��
���������⿼��ѧ������ԭ�ӽṹʾ��ͼ���ص㣬���ṩ����Ϣ���з������⣬�������Ӧ�õ�������

��ϰ��ϵ�д�
 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�
�����Ŀ

 ��ʾ����Ԫ�ط���ΪBr����ͼ��X=
��ʾ����Ԫ�ط���ΪBr����ͼ��X=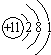







 ��1��������ԭ�ӵĺ˵����x=
��1��������ԭ�ӵĺ˵����x=




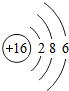



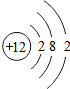
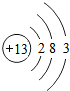


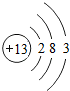 ��ʾ����
��ʾ����