��Ŀ����
ú�ǡ���ҵ��ʳ����úȼ��ʱ�����������������������ͬʱ�������ɴ�������Ⱦ�Ϊ�˼���ú��ȼ�նԻ�������Ⱦ����úת��Ϊ����ֵ�����ȼ�ϣ������¼��ִ�ʩ����1����ú�������õĽ�̿�ڸ�����������ˮ������Ӧ���õ�ˮú������Ҫ�ɷ���CO��H2�����÷�Ӧ�Ļ�ѧ����ʽΪ ��
��2������ˮú���е�CO��H2�ڼ��Ⱥʹ��������·�Ӧ�������Ƶ�Һ��ȼ�ϼ״���CH3OH�����÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3������ˮú���е�CO��H2�ڼ��Ⱥʹ��������·�Ӧ��Ҳ�����Ƶ�����ȼ�ϼ��飨CH4����ͬʱ����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4��������ˮ����ͨ���ú���ϣ���ûϨ�𣬷����������شڳ��ܸߵĻ��������������һ���� ��
���𰸡���������1��������Ϣ����̿�ڸ�����������ˮ������Ӧ���õ�ˮú������Ҫ�ɷ���CO��H2����д�ɻ�ѧ��Ӧʽ��
��2��������Ϣ��CO��H2�ڼ��Ⱥʹ��������·�Ӧ�������Ƶ�Һ��ȼ�ϼ״���CH3OH����д����ѧ��Ӧʽ��
��3��������Ϣ��CO��H2�ڼ��Ⱥʹ��������·�Ӧ��Ҳ�����Ƶ�����ȼ�ϼ��飨CH4����ͬʱ����ˮ��д����ѧ��Ӧʽ��
��4������ȼ����Ҫͬʱ���������������ٿ�ȼ�������������۴ﵽȼ�����������¶ȼ��Ż�㣩����ú����������ˮ�����ȷ�Ӧ������⣮
����⣺��1����Ϊ����̿�ڸ�����������ˮ������Ӧ���õ�ˮú������Ҫ�ɷ���CO��H2�������Ի�ѧ��Ӧʽ�ǣ�C+H2O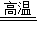 CO+H2�����C+H2O
CO+H2�����C+H2O 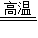 CO+H2��
CO+H2��
��2����Ϊ��CO��H2�ڼ��Ⱥʹ��������·�Ӧ�������Ƶ�Һ��ȼ�ϼ״���CH3OH�����ʷ���ʽΪ��CO+2H2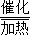 CH3OH�����CO+2H2
CH3OH�����CO+2H2 CH3OH��
CH3OH��
��3����Ϊ��CO��H2�ڼ��Ⱥʹ��������·�Ӧ��Ҳ�����Ƶ�����ȼ�ϼ��飨CH4����ͬʱ����ˮ���ʷ���ʽΪ��CO+3H2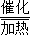 CH4+H2O�����CO+3H2
CH4+H2O�����CO+3H2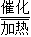 CH4+H2O��
CH4+H2O��
��4�����ȵ�̼��ˮ��Ӧ����ˮú��C+H2O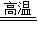 CO+H2�����»������������ˮú���ķ�Ӧ�����ȷ�Ӧ��
CO+H2�����»������������ˮú���ķ�Ӧ�����ȷ�Ӧ��
������ȵ�̼��ˮ��Ӧ����ˮú����
���������⿼�黯ѧ��Ӧʽ����д��ͬѧ�Dz���Ҫ��ס�α��еij�����ѧ��Ӧʽ��ҲҪ�ܹ������ṩ����Ϣ��д��ѧ��Ӧʽ��
��2��������Ϣ��CO��H2�ڼ��Ⱥʹ��������·�Ӧ�������Ƶ�Һ��ȼ�ϼ״���CH3OH����д����ѧ��Ӧʽ��
��3��������Ϣ��CO��H2�ڼ��Ⱥʹ��������·�Ӧ��Ҳ�����Ƶ�����ȼ�ϼ��飨CH4����ͬʱ����ˮ��д����ѧ��Ӧʽ��
��4������ȼ����Ҫͬʱ���������������ٿ�ȼ�������������۴ﵽȼ�����������¶ȼ��Ż�㣩����ú����������ˮ�����ȷ�Ӧ������⣮
����⣺��1����Ϊ����̿�ڸ�����������ˮ������Ӧ���õ�ˮú������Ҫ�ɷ���CO��H2�������Ի�ѧ��Ӧʽ�ǣ�C+H2O
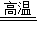 CO+H2�����C+H2O
CO+H2�����C+H2O 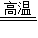 CO+H2��
CO+H2����2����Ϊ��CO��H2�ڼ��Ⱥʹ��������·�Ӧ�������Ƶ�Һ��ȼ�ϼ״���CH3OH�����ʷ���ʽΪ��CO+2H2
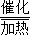 CH3OH�����CO+2H2
CH3OH�����CO+2H2 CH3OH��
CH3OH����3����Ϊ��CO��H2�ڼ��Ⱥʹ��������·�Ӧ��Ҳ�����Ƶ�����ȼ�ϼ��飨CH4����ͬʱ����ˮ���ʷ���ʽΪ��CO+3H2
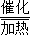 CH4+H2O�����CO+3H2
CH4+H2O�����CO+3H2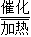 CH4+H2O��
CH4+H2O����4�����ȵ�̼��ˮ��Ӧ����ˮú��C+H2O
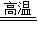 CO+H2�����»������������ˮú���ķ�Ӧ�����ȷ�Ӧ��
CO+H2�����»������������ˮú���ķ�Ӧ�����ȷ�Ӧ��������ȵ�̼��ˮ��Ӧ����ˮú����
���������⿼�黯ѧ��Ӧʽ����д��ͬѧ�Dz���Ҫ��ס�α��еij�����ѧ��Ӧʽ��ҲҪ�ܹ������ṩ����Ϣ��д��ѧ��Ӧʽ��

��ϰ��ϵ�д�
�����Ŀ