ƒøƒ⁄»ð
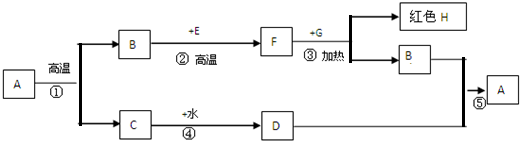
A «º¶µ∞ø«°¢±¥ø«°¢ ت“ صƒ÷˜“™≥…∑÷£¨B°¢F «‘™Àÿ◊È≥…œýÕ¨µƒ∆¯Ã£¨E°¢G «∫⁄…´πÃã¨∏˘æ𜬡–πÿœµ£®≤ø∑÷≤˙ŒÔ¬‘»•£©£¨ÕÍ≥…œ¬¡–∏˜Ã‚°£
|
|
|
|
|
|
|

|


|

|


|

|

|
|


|
|

|
£®1£©CµƒÀ◊√˚ « £¨EµƒªØ—ß Ω £ª
£®2£©¢€µƒªØ—ß∑Ω≥Ã Ω « £¨
¿˚”√F’‚“ª–‘÷ ø… £¨‘⁄◊ˆ¥À µ—È ±£¨≥˝ºÏ≤È◊∞÷√∆¯√Ж‘Õ‚£¨º”»»«∞”¶ £¨ƒøµƒ « £ª
£®3£©¢ÐµƒªØ—ß∑Ω≥Ã Ω £¨
∑¥”¶µƒª˘±æ¿ý–Õ « £¨∏√∑¥”¶ « £®ÃÓ°∞Œ¸»»°±ªÚ°∞∑≈»»°±£©∑¥”¶°£
|
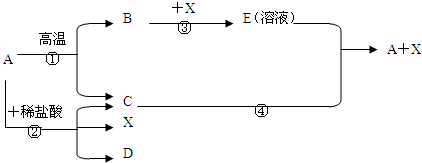
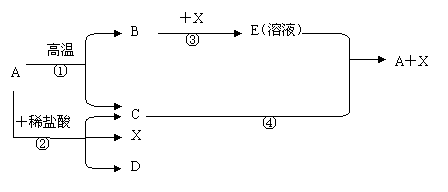
 £®2£© CO+CuO Cu+CO2 £ª
£®2£© CO+CuO Cu+CO2 £ª
ø…“±¡∂Ω Ù£ªœ»Õ®»Î“ª∂Œ ±º‰CO£ª∏œ◊þ◊∞÷√÷–µƒø’∆¯£¨±Ð√‚CO≤ª¥ø∂¯±¨’®
 £®3£©CaO+H2O Ca(OH)2 £ªªØ∫œ∑¥”¶£ª∑≈»»
£®3£©CaO+H2O Ca(OH)2 £ªªØ∫œ∑¥”¶£ª∑≈»»

 ΩÃ≤ƒ»´Ω‚◊÷¥ æ‰∆™œµ¡–¥∞∏
ΩÃ≤ƒ»´Ω‚◊÷¥ æ‰∆™œµ¡–¥∞∏