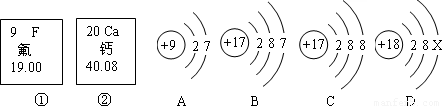
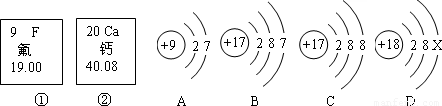
��Ŀ����
��2008?��������ͼ�еĢ١����Ƿ�Ԫ�ء���Ԫ����Ԫ�����ڱ��е���Ϣ��A��B��C��D���������ӵĽṹʾ��ͼ��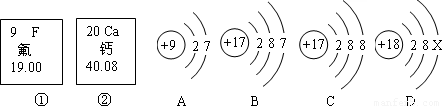
����ش�
��1����Ԫ�ص����ԭ������Ϊ����Ԫ�ص�ԭ������Ϊ��
��2��X=��
��3��A��B��C��D����ͬ��Ԫ�ص������ǣ�����ţ���
��4��A���ӵĻ�ѧ������B��C��D����һ�����ӵĻ�ѧ�������ƣ�����ţ���
��5��2008��5�£���ʡij�ط���������ᣨHF��й©��������Ա����ʯ�Ҷ�й©����д�������д����ѧ����ʽ��
���𰸡���������1�����ݷ�Ԫ�ء���Ԫ����Ԫ�����ڱ��е���Ϣ���н��
��2��������ԭ���У�ԭ������=������=���������=�˵���������н��
��3������Ԫ���Ǿ�����ͬ�˵����������������������һ��ԭ�ӵ��ܳƣ���ͬ��Ԫ��֮��ı�����������������ͬ�������жϽ��
��4������Ԫ�صĻ�ѧ���ʸ�����ԭ�ӵ�����������Ŀ��ϵ�dz����У�������������ͬ��Ԫ�ػ�ѧ�������ƣ����н��
��5��������д��ѧ����ʽ��Ҫ��д���䣬ע���ȣ�д����Ӧ����ʽ��
����⣺��1�����ݷ�Ԫ�ء���Ԫ����Ԫ�����ڱ��е���Ϣ��֪����Ԫ�ص����ԭ������Ϊ 19.00������Ԫ�ص�ԭ������Ϊ��20��
�ʴ�Ϊ��19.00�� 20��
��2��������ԭ���У�ԭ������=������=���������=�˵���������У�2+8+x=18������x=8���ʴ�Ϊ��8��
��3������Ԫ���Ǿ�����ͬ�˵����������������������һ��ԭ�ӵ��ܳƣ���ͬ��Ԫ��֮��ı�����������������ͬ����BC����������ͬ��Ϊͬ��Ԫ�أ��ʴ�Ϊ��BC��
��4������Ԫ�ص�������������ͬ��Ԫ�ػ�ѧ�������ƣ���A��B��������������ͬ���ʴ�Ϊ��B��
��5����������ᣨHF��й©����ʯ�ҽ��д���D ��Ӧ����ʽΪ��Ca��OH��2+2HF=CaF2��+2H2O��
�ʴ�Ϊ��Ca��OH��2+2HF=CaF2��+2H2O��
���������⿼��ѧ������ԭ���У�ԭ����������������������������˵����֮��Ĺ�ϵ��Ԫ�ص���������������Ԫ�صĻ�ѧ���ʵ����������գ���ע����д��ѧ����ʽ��Ҫ��
��2��������ԭ���У�ԭ������=������=���������=�˵���������н��
��3������Ԫ���Ǿ�����ͬ�˵����������������������һ��ԭ�ӵ��ܳƣ���ͬ��Ԫ��֮��ı�����������������ͬ�������жϽ��
��4������Ԫ�صĻ�ѧ���ʸ�����ԭ�ӵ�����������Ŀ��ϵ�dz����У�������������ͬ��Ԫ�ػ�ѧ�������ƣ����н��
��5��������д��ѧ����ʽ��Ҫ��д���䣬ע���ȣ�д����Ӧ����ʽ��
����⣺��1�����ݷ�Ԫ�ء���Ԫ����Ԫ�����ڱ��е���Ϣ��֪����Ԫ�ص����ԭ������Ϊ 19.00������Ԫ�ص�ԭ������Ϊ��20��
�ʴ�Ϊ��19.00�� 20��
��2��������ԭ���У�ԭ������=������=���������=�˵���������У�2+8+x=18������x=8���ʴ�Ϊ��8��
��3������Ԫ���Ǿ�����ͬ�˵����������������������һ��ԭ�ӵ��ܳƣ���ͬ��Ԫ��֮��ı�����������������ͬ����BC����������ͬ��Ϊͬ��Ԫ�أ��ʴ�Ϊ��BC��
��4������Ԫ�ص�������������ͬ��Ԫ�ػ�ѧ�������ƣ���A��B��������������ͬ���ʴ�Ϊ��B��
��5����������ᣨHF��й©����ʯ�ҽ��д���D ��Ӧ����ʽΪ��Ca��OH��2+2HF=CaF2��+2H2O��
�ʴ�Ϊ��Ca��OH��2+2HF=CaF2��+2H2O��
���������⿼��ѧ������ԭ���У�ԭ����������������������������˵����֮��Ĺ�ϵ��Ԫ�ص���������������Ԫ�صĻ�ѧ���ʵ����������գ���ע����д��ѧ����ʽ��Ҫ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��2008?��������ͼ��Ϊ�Ȼ��ơ�̼���ƣ��׳ƴ����ˮ�е��ܽ�����ߣ�
��2008?��������ͼ��Ϊ�Ȼ��ơ�̼���ƣ��׳ƴ����ˮ�е��ܽ�����ߣ�