��Ŀ����
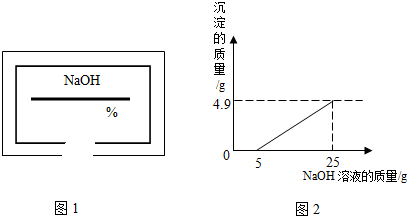
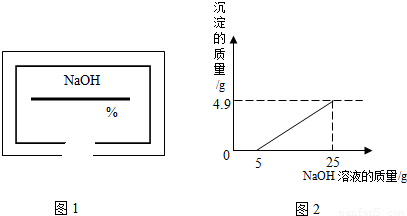
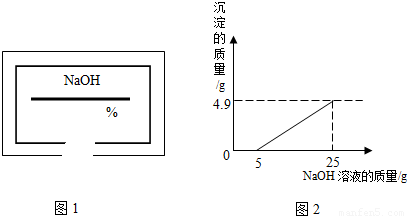
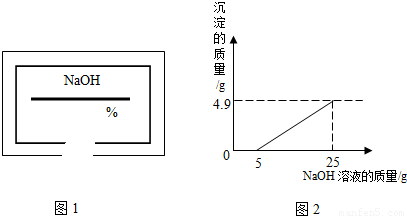
С��ͬѧ��ʵ���ҿ�������������ͭ��ϡ���ᷴӦ��ʵ�����ʵ��������һƿ��ǩ��ȱ����ͼ1��ʾ����NaOH��Һ��Ϊ�˲ⶨ����Һ������������������������������ͭ��ϡ���ᷴӦ��ķ�Һ��������Һ���ˣ�Ȼ��ȡ100g��Һ�������μӴ�NaOH��Һ������NaOH��Һ�����������ɳ��������Ĺ�ϵ��ͼ2��ʾ��
��1���ڼ�������������Һ�Ĺ����У���ʼʱû�з��ֳ������ɣ�˵����Һ�е����ʳ�����CuSO4�⣬������________��д��ѧʽ����
��2������100g��Һ��CuSO4��������
��3�����������������Һ�����ʵ�����������
�⣺��1���ڼ�������������Һ�Ĺ����У���ʼʱû�з��ֳ������ɣ�����Ϊ����������Һ����ϡ���ᷴӦ��˵������ͭ��ϡ���ᷴӦ��ϡ������ʣ�࣮�ʴ�Ϊ��H2SO4��
��2���⣺��100g��Һ��CuSO4������Ϊx����CuSO4��Ӧ����4.9g������NaOH����Ϊy��
CuSO4+2NaOH=Cu��OH��2��+Na2SO4
160----80-------98
x------y-------4.9g
��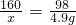
��֮�ã�x=8g��
��3��
��֮�ã�y=4g��
������������Һ�����ʵ���������=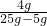 ��100%=20%��
��100%=20%��
�𣺣�2��100g��Һ��CuSO4��������8g����3��������������Һ�����ʵ�����������20%��
��������1���ڼ�������������Һ�Ĺ����У���ʼʱû�з��ֳ������ɣ�����Ϊ����������Һ����ϡ���ᷴӦ��˵������ͭ��ϡ���ᷴӦ��ϡ������ʣ�ࣻ
��2����ͼʾ��֪����������������Һ��25gʱ�����ɳ���������Ϊ4.9g����������ͭ���������Ʒ�Ӧ�Ļ�ѧ����ʽ�����ɳ������������г�����ʽ���Ϳɼ����100g��Һ��CuSO4��������
��3�����ݻ�ѧ����ʽ�г�����ʽ���Ϳɼ��������CuSO4��Ӧ����4.9g������NaOH������Ȼ�����������������=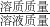 ��100%���㼴�ɣ�
��100%���㼴�ɣ�
������������Ҫ����ѧ�����û�ѧ����ʽ����������������ʽ���м����������
��2���⣺��100g��Һ��CuSO4������Ϊx����CuSO4��Ӧ����4.9g������NaOH����Ϊy��
CuSO4+2NaOH=Cu��OH��2��+Na2SO4
160----80-------98
x------y-------4.9g
��
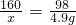
��֮�ã�x=8g��
��3��

��֮�ã�y=4g��
������������Һ�����ʵ���������=
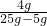 ��100%=20%��
��100%=20%���𣺣�2��100g��Һ��CuSO4��������8g����3��������������Һ�����ʵ�����������20%��
��������1���ڼ�������������Һ�Ĺ����У���ʼʱû�з��ֳ������ɣ�����Ϊ����������Һ����ϡ���ᷴӦ��˵������ͭ��ϡ���ᷴӦ��ϡ������ʣ�ࣻ
��2����ͼʾ��֪����������������Һ��25gʱ�����ɳ���������Ϊ4.9g����������ͭ���������Ʒ�Ӧ�Ļ�ѧ����ʽ�����ɳ������������г�����ʽ���Ϳɼ����100g��Һ��CuSO4��������
��3�����ݻ�ѧ����ʽ�г�����ʽ���Ϳɼ��������CuSO4��Ӧ����4.9g������NaOH������Ȼ�����������������=
 ��100%���㼴�ɣ�
��100%���㼴�ɣ�������������Ҫ����ѧ�����û�ѧ����ʽ����������������ʽ���м����������

��ϰ��ϵ�д�
 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д�
�����Ŀ