��Ŀ����
��5�֣��������Ҵ����ͣ����ƹ��ʹ�ý��Ỻ��ʯ����Դ���š��Ҵ�����ѧʽΪC2H��OH����ȫȼ������CO2��H2O��
������д���Ҵ���ȫȼ�յĻ�ѧ����ʽ����������
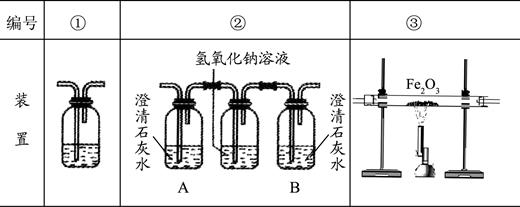
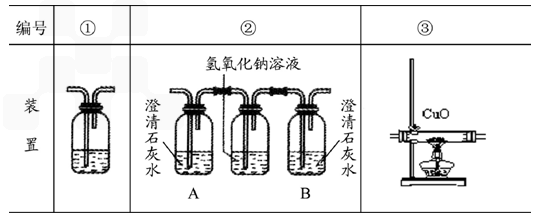
�������Ҵ�ȼ��ʱ������������㣬���ܻ���CO���ɣ�������װ��ȷ֤�Ҵ�ȼ�ղ�����CO��CO2��Ӧ���Ҵ�ȼ�ղ�������ͨ������������������˳����װ�ñ�ţ���������
��ȷ���Ӻ��װ���Դ��ڲ��㣬��ָ���䲻��֮����������
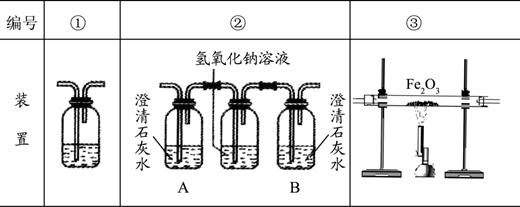
������װ�â��У���B��ƿ��ʢ�г����ʯ��ˮ�������÷ֱ�����������
������д��װ�â�����ʢ��Һ���Ƽ�����Һ��������������
��1��C2H5OH+3O2 2CO2+3H2O
2CO2+3H2O
��2���ڢۢ� û��β������װ��
��3��A����ȼ�ղ������ж�����̼��B֤��ȼ�ղ����еĶ�����̼�Ƿ�����������Һ��ȫ���ա�
��4�������ʯ��ˮ��������֤�����з�Ӧ�ж�����̼���ɣ��Ӷ�˵��ԭȼ�ղ�������һ����̼��
��������
����������������Ҵ���ȫȼ�յĻ�ѧ����ʽ��C2H5OH+3O2 2CO2+3H2O��
2CO2+3H2O��
��2��Ϊȷ֤�Ҵ�ȼ�ղ�����CO��CO2���ȵü����ж�����̼�������ȥ���ټ��麬��һ����̼����װ������Ϊ���ڢۢ٣�������װ��û��β������װ�ã�������װ�û��в��㣻
������װ�â��У���B��ƿ��ʢ�г����ʯ��ˮ��A����ȼ�ղ������ж�����̼��B֤��ȼ�ղ����еĶ�����̼�Ƿ�����������Һ��ȫ���ա�
������װ�â�����ʢ��Һ�dz����ʯ��ˮ������Һ��������֤�����з�Ӧ�ж�����̼���ɣ��Ӷ�˵��ԭȼ�ղ�������һ����̼��
���㣺��ѧ����ʽ�������ƶ�����飻������̼�����ʣ�һ����̼�����ʡ�
������������Ĺؼ���ҪŪ���ȼ�ղ�����CO��CO2�ļ���˳��

 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�