��Ŀ����
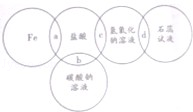
��������(ClO2)����������ˮ�������Դ���Ϊԭ������ClO2�Ĺ�����Ҫ�������ٴ��ξ��ƣ��ڵ������NaCl��Һ����ClO2����ȡ��������������ͼ�����з�Ӧ����ȡClO2�Ļ�ѧ����ʽΪ2NaClO3+4HCl=2ClO2��+Cl2��+2NaCl+2H2O��

��1���Լ�X��________(�ѧʽ)������A��������_________����������HCl��Ϊ��������ʱ�������⣬���ܳ�ȥ��Һ�е��������ӣ���д���������������кͷ�Ӧ�Ļ�ѧ����ʽ��________________________________��
��2��������ɷ�Ӧ��Ļ�ѧ����ʽ: _____NaCl+_____H2O ______________��
______________��
��3��Ϊ��߾���Ч�棬���������г���H2��Cl2�Ƴ�HCl��������⣬����ѭ��ʹ�õ�����Y��_________��

��1���Լ�X��________(�ѧʽ)������A��������_________����������HCl��Ϊ��������ʱ�������⣬���ܳ�ȥ��Һ�е��������ӣ���д���������������кͷ�Ӧ�Ļ�ѧ����ʽ��________________________________��
��2��������ɷ�Ӧ��Ļ�ѧ����ʽ: _____NaCl+_____H2O
 ______________��
______________����3��Ϊ��߾���Ч�棬���������г���H2��Cl2�Ƴ�HCl��������⣬����ѭ��ʹ�õ�����Y��_________��
��1��Na2CO3 ���� NaOH+HCl=NaCl+H2O ��2��NaCl+3H2O NaClO3+3H2��
NaClO3+3H2��
��3���Ȼ���
 NaClO3+3H2��
NaClO3+3H2����3���Ȼ���
��1������ˮ��Ca2+��Mg2+�Ŀ��Էֱ���������Na2CO3��NaOH�γɳ������˵�����X��Na2CO3������A�ǹ��ˣ�������Na2CO3��NaOH���Լ��������������ȥ������������������ķ�Ӧ�����кͷ�Ӧ����2��������ȡClO2�Ļ�ѧ����ʽ��֪���ʳ��ˮ�ķ�Ӧ��IJ�����H2��NaClO3����3��NaClO3�ٺ�HCl������Ӧ��2NaClO3+4HCl=2ClO2��+Cl2��+2NaCl+2H2O��Cl2���Ժ�H2��Ӧ�Ƴ� HCl������ã�NaCl(Y����)Ҳ��ѭ��ʹ�á�

��ϰ��ϵ�д�
 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�
�����Ŀ