��Ŀ����
��һ��������Mg��Al�Ͻ�Ͷ��100gһ��Ũ�ȵ������У��Ͻ�ȫ���ܽ⣬��������Һ�еμ�NaOH��Һ�����������ɳ��������������NaOH��������ϵ��ͼ��ʾ��(��֪��Al(OH)3��������NaOH��Һ��Ӧ����Ӧ����ʽΪ��Al(OH)3+NaOH=NaAlO2+2H2O��Mg(OH)2����������NaOH��Һ��Ӧ��)��

(1)Al(OH)3������Ϊ_____________��
(2)ԭ�Ͻ���Mg��Al��������Ϊ______(��Ϊ���������)��
(3)�����������������Ϊ_____��
��ϰ��ϵ�д�
 �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д� �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�
�����Ŀ
������ڲ�ͬ�¶��µ��ܽ�������
�¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 |
�ܽ��/g | 13.3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.8 |
��1��30��ʱ����15.8g�������ȫ�ܽ���50gˮ�У������������Һ����������Ϊ_____��
��2�����裨1�����õ���Һ��������Ҫ����_____������ز��ܱ��͡�


 һ���¶��£��͵��������Һ�в��ϼ�������ع���
һ���¶��£��͵��������Һ�в��ϼ�������ع���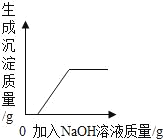 �����������ͭ�����Һ�еμӹ���������������Һ
�����������ͭ�����Һ�еμӹ���������������Һ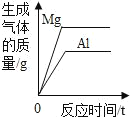 ����������þ�ۺ����۷ֱ���������Ũ�ȵ�ϡ���ᷴӦ
����������þ�ۺ����۷ֱ���������Ũ�ȵ�ϡ���ᷴӦ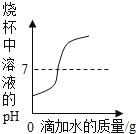 ��ʢ��һ����ϡ������ձ��в��ϼ�ˮϡ��
��ʢ��һ����ϡ������ձ��в��ϼ�ˮϡ��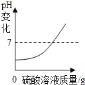 ������ˮ B.
������ˮ B.  ������п
������п ����������������Һ D.
����������������Һ D.  �������Ȼ�����Һ
�������Ȼ�����Һ