��Ŀ����
2008��2�£��ձ����ƴ��й��䶳���Ӽ��������е�η���������ձ����۷�����ʹ�õ�ɱ������е�η�ڼ�����Һ�пɷֽ�Ϊ�����������C2H6O4S���Ͷ�����ȩ��Cl2CHCHO����
��1��������ȩ����Ԫ�ص���������Ϊ______����ȷ��0.1%��
��2������������ڿ�������ȫȼ�������ɶ�����̼����������һ�ֳ�����Һ̬�������÷�Ӧ�Ļ�ѧ����ʽΪ______��
��3���������������ˮ������Ӧ��C2H6O4S+2H2O�TH2SO4+2X����X�Ļ�ѧʽΪ______��
�⣺��1��������ȩ����Ԫ�ص���������=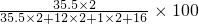 %=62.8%��
%=62.8%��
��2������������ڿ�������ȫȼ�������ɶ�����̼����������ˮ����Ӧ�Ļ�ѧ����ʽΪ��2C2H6O4+5O2 4CO2+SO2+6H2O��
4CO2+SO2+6H2O��
��3�����������غ㶨�ɿ�֪��ѧ��Ӧǰ��ԭ�ӵ�����������䣬��Ӧǰ��2��̼ԭ�ӡ�10����ԭ�ӡ�6����ԭ�Ӻ�1����ԭ�ӣ����Է�Ӧ��2x��Ӧ����2��̼ԭ�ӡ�8����ԭ�ӡ�2����ԭ�ӣ���x�Ļ�ѧʽΪ��CH4O��
�ʴ�Ϊ��
��1��62.8%��
��2��2C2H6O4+5O2 4CO2+SO2+6H2O��
4CO2+SO2+6H2O��
��3��CH4O��
��������1�����ݻ�������ijԪ�ص���������= ��100%���н��
��100%����
��2�����ݷ�Ӧ��������Լ���Ӧ����д����Ӧ�Ļ�ѧ����ʽ��
��3�����������غ㶨�ɿ�֪����ѧ��Ӧǰ��ԭ�ӵ��������������н��
���������⿼�����ʵĻ�ѧʽ�Լ������غ㶨�ɵĺ��壬�ص��Ƕ������غ㶨�ɵ��۽��ͣ�ע��֪ʶ��Ǩ��ʹ�ã�ע��ѧ��������ѵ����
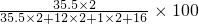 %=62.8%��
%=62.8%����2������������ڿ�������ȫȼ�������ɶ�����̼����������ˮ����Ӧ�Ļ�ѧ����ʽΪ��2C2H6O4+5O2
 4CO2+SO2+6H2O��
4CO2+SO2+6H2O����3�����������غ㶨�ɿ�֪��ѧ��Ӧǰ��ԭ�ӵ�����������䣬��Ӧǰ��2��̼ԭ�ӡ�10����ԭ�ӡ�6����ԭ�Ӻ�1����ԭ�ӣ����Է�Ӧ��2x��Ӧ����2��̼ԭ�ӡ�8����ԭ�ӡ�2����ԭ�ӣ���x�Ļ�ѧʽΪ��CH4O��
�ʴ�Ϊ��
��1��62.8%��
��2��2C2H6O4+5O2
 4CO2+SO2+6H2O��
4CO2+SO2+6H2O����3��CH4O��
��������1�����ݻ�������ijԪ�ص���������=
 ��100%����
��100%���н����2�����ݷ�Ӧ��������Լ���Ӧ����д����Ӧ�Ļ�ѧ����ʽ��
��3�����������غ㶨�ɿ�֪����ѧ��Ӧǰ��ԭ�ӵ��������������н��
���������⿼�����ʵĻ�ѧʽ�Լ������غ㶨�ɵĺ��壬�ص��Ƕ������غ㶨�ɵ��۽��ͣ�ע��֪ʶ��Ǩ��ʹ�ã�ע��ѧ��������ѵ����

��ϰ��ϵ�д�
 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д� ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д� ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д�
�����Ŀ