��Ŀ����
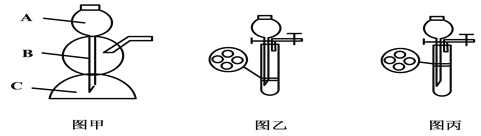
����Ŀ����������װ�ö�������ʵ������ȡ������̼

��1��д��ʵ������ȡ������̼�Ļ�ѧ����ʽ:____________________��
��2������A��������__________________
��3������ͼ2װȡ��ȡ������̼ʱ������©�����¶˹ܿڱ����û����Һ�У�������_____________��
��4��ͼ����ͼ3װ����ȣ�����ͼ��װ������ȡ������̼ʱ��Ҫ�IJ�����_______��дһ��������
��5����ѧ��ȤС��Ϊ�˲ⶨʯ��ʯ��Ʒ��̼��Ƶ�����������ȡһ������ʯ��ʯ��Ʒ����20gϡ�����4�μ�����Ʒ������Ʒ�г�̼����⣬����ɷֲ������ᷴӦ��Ҳ������ˮ������ַ�Ӧ�����ˡ�ǧ�ٵȲ��������������������±�
ϡ������ʣ���������� | |
��һ�μ���5g | 1��5g |
�ڶ��μ���59 | 1��0g |
�������59 | 0��5g |
���Ĵμ���59 | 0��3g |
��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ_________________
��ԭϡ���������ʵ���������Ϊ������д�����������
���𰸡���1��CaCO3+2HCl==CaCl2+H2O+CO2����2����ƿ����3����ֹ����������ӳ���©�����ݳ�;��4�����ܿ��Ʒ�Ӧ�ķ�����ֹͣ����5����85%����7��3%
��������
���������������ѧ֪ʶ��������Ϣ֪����1��ʵ������ȡ������̼�Ļ�ѧ����ʽ:
CaCO3+2HCl==CaCl2+H2O+CO2������2������A����������ƿ����3������ͼ��װȡ��ȡ������̼ʱ������©�����¶˹ܿڱ����û����Һ�У���������ֹ����������ӳ���©�����ݳ�;��4��ͼ����ͼ��װ����ȣ�����ͼ��װ������ȡ������̼ʱ��Ҫ�IJ��������ܿ��Ʒ�Ӧ�ķ�����ֹͣ������ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ����2.0g��0.3g�w��2g��100����85��.��ԭϡ���������ʵ���������Ϊ��7��3%��
�������һ����̼��Ʒ�Ӧ���Ȼ�������Ϊxg
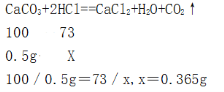
ԭϡ���������ʵ���������Ϊ��0.365g��5g��100����7��3%
���â�ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ85%����ԭϡ���������ʵ���������Ϊ
����������ʵ������ȡ������̼װ�ã�ԭ�����������йؼ��㡣