��Ŀ����
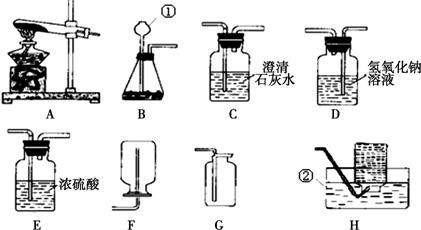
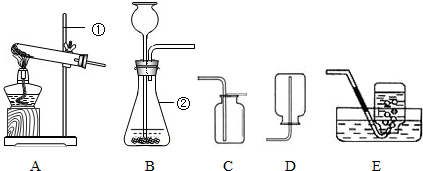
��������װ��ͼ���ش�������⡣

(1)���ü�������صķ�����ȡ��������Ӧ�Ļ�ѧ����ʽΪ��________________________________________________________________________��
����װ�ÿ�ѡ��ͼ�е�________(����)���ռ�����ѡ��ͼ�е�Cװ�ã���Dװ����ȣ�Cװ�õ��ŵ����ռ���������________________��
(2)���÷ֽ����������Һ�ķ�����ȡ����������װ�ÿ�ѡ��ͼ�е�Bװ�á�
�����÷�Ӧ��MnO2����������MnO2Ӧ��װ��Bװ�õ�__________(����������)�У���Ӧ�Ļ�ѧ����ʽΪ��________________________________________________________________________��
���±��Ƿֽ���ͬŨ�ȵĹ���������Һʱѡ�ò�ͬ�������õ���ʵ�����ݣ�
| ���� (������Ϊ0.3 g) | �������� | CuCl2 | MnO2 | CuO |
| �ռ�1 L������ ���ʱ��/s | 42 | 650 | 18 | 300 |
���������ݿɵó�һ�����ۣ�_________________________________________________��
(1)2KClO3 2KCl��3O2����A���Ƚϴ���
2KCl��3O2����A���Ƚϴ���
(2)����ƿ��2H2O2 2H2O��O2��
2H2O��O2��
�������������䣬��ͬ�����Էֽ����������Һ�Ĵ�����ǿ��ΪMnO2>��������>CuO>CuCl2