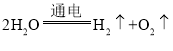��Ŀ����
����Ŀ��̼��̼�Ļ������ǻ�ѧ�����������Ӵ�ļ��塣
(1)����̿��������Ч��������еİ���������ȩ���к����壬ԭ���ǻ���̿����______�ԡ�����N95���ֵ���Ҫԭ���Ǿ۱�ϩ[(C3H6)n]���۱�ϩ_____(����������������������)�л��ϳɲ��ϡ�
(2)���Ż�ʯȼ�ϵĴ���ʹ�ô������ǻ�������Ⱦ����Դ�Ŀݽߵ����⡣�����Ѿ�ȫ���ƹ�ʹ�ó����Ҵ����͡��Ҵ�ȼ�յĻ�ѧ����ʽ��_______��
(3)�ҹ�������չ��̼���ã�����̼�����ν����̼�������ǽϵ͵�______(�ѧʽ)�ŷš�
(4)��ͼ����Ȼ���е�̼����ѭ��ʾ��ͼ������˵����һ����ȷ����_______(�����)��

A �����е�O2Խ��Խ�ã�CO2Խ��Խ��
B ��ʯȼ��ȼ��ʱ��ѧ�ܿ���ת��Ϊ���ܺ���
C ��ɫֲ����������̣����漰̼ѭ�������漰��ѭ��
���𰸡����� ���� C2H5OH+3O2![]() 3H2O+2CO2 CO2 BC
3H2O+2CO2 CO2 BC
��������
��1������̿���������ԣ�����Ч��������еİ���������ȩ���к����壬�۱�ϩ��̼Ԫ�أ������л��ϳɲ��ϣ���������ԣ����ڡ�
��2���Ҵ�ȼ�����ɶ�����̼��ˮ����ѧ����ʽΪC2H5OH+3O2![]() 3H2O+2CO2�����C2H5OH+3O2
3H2O+2CO2�����C2H5OH+3O2![]() 3H2O+2CO2��
3H2O+2CO2��
��3����̼����ָ���ǽϵ͵Ķ�����̼�ŷ��������CO2��
��4��A�������ܹ���������������̼�ܰ���ֲ�������ã����Ǵ����е�O2Խ��Խ�ã�CO2Խ��Խ��A����
B����ʯȼ��ȼ��ʱ������ȣ���ѧ�ܿ���ת��Ϊ���ܺ��ܣ���ȷ��
C����ɫֲ����������̣����й���������ն�����̼���ͷ����������������������������ͷŶ�����̼�����漰̼ѭ�������漰��ѭ������ȷ��
��ѡ��BC��

����Ŀ�����б��е���������Ӧ�Ļ�ѧ����ʽ��������Ӧ�������Ͷ���ȷ���ǣ�������
ѡ�� | ���� | ��ѧ��Ӧ����ʽ | ��Ӧ���� |
A | ���ˮ |
| �ֽⷴӦ |
B | ̽��һ����̼�Ļ�ԭ�� |
| �û���Ӧ |
C | �����γɵ�ԭ�� |
| ���Ϸ�Ӧ |
D | ����ͭ��Һ�еμ�����������Һ |
| ���ֽⷴӦ |
A.AB.BC.CD.D