��Ŀ����
 �������г����������й�����̽����
�������г����������й�����̽����
�����ʯ��ʱ��������ͭ��ʱ�����̶���������ʱ����ǰ���������и��������Ǻ������ҹ�ҵ�;��÷�չ�ı�־���ɼ��������ϳ�������һֱ���㷺��Ӧ���ţ��Խ������ϵ�̽��Ҫ�õ����ѧ֪ʶ��
��1��������֮�����ɣ�1������2���Ŀո��Դ�Ϊ����������д������������֮���
��Ŀǰ�������������ߵĽ�����______��
�ڵؿ��к������Ľ�����______��
��______����______��
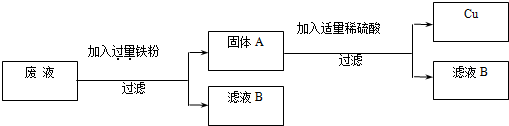
��2�������Ϊ�����������á���ij���ķ�Һ�к���H2SO4��CuSO4��Ϊ�˷�ֹ�����CuSO4��Ⱦ��������������ú����ķ�������ս���ͭ��ͬʱ������Ҫ�Ļ���ԭ�������������壬���ԭ����ͼ��ʾ��
�������������Ʒ���д���йػ�ѧ����ʽ��______��______��
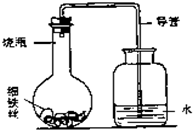
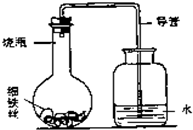
��3������ʴ������̽������ijѧ����A��B��C��D��ֻС��ƿ�зֱ��������ϸ��˿������ʳ��ˮ��ϸ��˿��������ˮ��ϸ��˿��ʳ��ˮ��ϸ��˿����ʹ��˿��ȫ��û��ʳ��ˮ�У�Ȼ��װ�����ͼ��ʾ������װ�ã�ÿ��һ��ʱ�����������ˮ�������ĸ߶ȣ�������±���������������Ϊ������ˮ�������ĸ߶�/cm����ʾ��
| ʱ��/Сʱ | 0 | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 |
| Aƿ��ʢ������˿�� | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bƿ��ʢմ��ʳ��ˮ����˿�� | 0 | 0.4 | 1.2 | 3.4 | 5.6 | 7.6 | 9.8 |
| Cƿ��ʢմ����ˮ����˿�� | 0 | 0 | 0 | 0.3 | 0.8 | 2.0 | 3.5 |
| Dƿ��ʢ��ȫ��û��ʳ��ˮ�е���˿�� | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
������ʵ���У�������������ɴ�С������˳��Ϊ����С��ƿ�ţ���______��
��Ӱ��������������У�______��
��4���������ķ�����������η�ֹ������г��ĸ�Ȧ����������ַ�����______��
��5����������ұ������ij�������ղ���4%���ʵ�����2240�֣��ʣ���Ҫ��Fe2O3��������Ϊ80%�ij�����ʯ���ٶ֣�
�⣺��1����Ŀǰ�������������ߵĽ����� �����ڵؿ��к������Ľ����� �������۵���͵Ľ����ǹ������۵���ߵĽ������٣�
��2��ij���ķ�Һ�к���H2SO4��CuSO4������������ۺ���������ͭ��Ӧ����ͭ�������������������ᷴӦ����������������������ѧ����ʽΪ
��3����������ʱ����������ʹƿ��ѹǿ���ͣ����ѹǿ��ˮѹ�뵼���ڣ�
��������������ˮ��ͬ�Ӵ��Ļ����������⣬���������Һ�������ٶȼӿ죮�������ʵ���У�������������ɴ�С������˳��Ϊ��B��C��A=D��
��Ӱ��������������У�ˮ��������������Һ�ٶ���죮
��4���ڸ�������Ϳˢ�����ͻ�������������ȸ��DZ���Ĥ�����Ը���������ˮ����ֹ�����⣮��������г���Ȧ�϶�һ�������Ϳ�Ϳ��Է�ֹ���⣮
��5���⣺����Ҫ��Fe2O380%�ij�����ʯΪx
2240t����1-4%��=x��80%��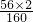 ��100%
��100%
x=3840t
�ʴ�Ϊ����1�����������������۵���͵Ľ����ǹ������۵���ߵĽ�������
��2��Fe+H2SO4 �TFeSO4+H2���� Fe+CuSO4�TCu+FeSO4
��3����������ʱ����������ʹƿ��ѹǿ���ͣ�
��B��C��A=D��
��ˮ��������������Һ�ٶ���죮��
��4����һ�������Ϳ��
��5��3840t
��������1�����ݽ���֮�����
��2���������Ļ�ѧ���ʷ���
��3������������������Լ����ⷽ������
��4����ֹ��������ķ����У��ڽ�������Ϳһ������ڽ��������һ��������ڽ�������Ϳһ���͵ȣ�
��5�����ݻ�ѧ�仯ǰ��Ԫ���������䣬�������������������������ʯ��������Ԫ��������ȣ�������һ������ϵ��������⣬���г�����ʯ����Ԫ������������ʯ��������������Ԫ�ص�������
���������⿼�������йص�֪ʶ����������֮����Ļ�ѧ���ʡ�������������Լ��й����ļ��㣬������֪ʶ���飮
��2��ij���ķ�Һ�к���H2SO4��CuSO4������������ۺ���������ͭ��Ӧ����ͭ�������������������ᷴӦ����������������������ѧ����ʽΪ
��3����������ʱ����������ʹƿ��ѹǿ���ͣ����ѹǿ��ˮѹ�뵼���ڣ�
��������������ˮ��ͬ�Ӵ��Ļ����������⣬���������Һ�������ٶȼӿ죮�������ʵ���У�������������ɴ�С������˳��Ϊ��B��C��A=D��
��Ӱ��������������У�ˮ��������������Һ�ٶ���죮
��4���ڸ�������Ϳˢ�����ͻ�������������ȸ��DZ���Ĥ�����Ը���������ˮ����ֹ�����⣮��������г���Ȧ�϶�һ�������Ϳ�Ϳ��Է�ֹ���⣮
��5���⣺����Ҫ��Fe2O380%�ij�����ʯΪx
2240t����1-4%��=x��80%��
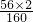 ��100%
��100%x=3840t
�ʴ�Ϊ����1�����������������۵���͵Ľ����ǹ������۵���ߵĽ�������
��2��Fe+H2SO4 �TFeSO4+H2���� Fe+CuSO4�TCu+FeSO4
��3����������ʱ����������ʹƿ��ѹǿ���ͣ�
��B��C��A=D��
��ˮ��������������Һ�ٶ���죮��
��4����һ�������Ϳ��
��5��3840t
��������1�����ݽ���֮�����
��2���������Ļ�ѧ���ʷ���
��3������������������Լ����ⷽ������
��4����ֹ��������ķ����У��ڽ�������Ϳһ������ڽ��������һ��������ڽ�������Ϳһ���͵ȣ�
��5�����ݻ�ѧ�仯ǰ��Ԫ���������䣬�������������������������ʯ��������Ԫ��������ȣ�������һ������ϵ��������⣬���г�����ʯ����Ԫ������������ʯ��������������Ԫ�ص�������
���������⿼�������йص�֪ʶ����������֮����Ļ�ѧ���ʡ�������������Լ��й����ļ��㣬������֪ʶ���飮

��ϰ��ϵ�д�
�����Ŀ