��Ŀ����
��2013?�麣���ֿ�����һ�����ʵİ�װ��ǩģ�����壬��ͬѧ����������̽����
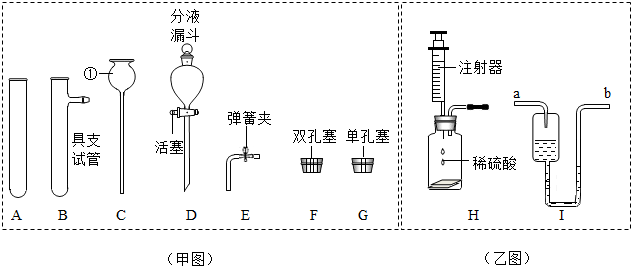
��1���������ò��������Ŀ����

��2��������±�����֪AgCl��BaSO4��������ˮ����İ�ɫ���壩��
��3�������������������õ��ʿ�����
��1���������ò��������Ŀ����
ʹ��Ʒ����ܽ�
ʹ��Ʒ����ܽ�
��
��2��������±�����֪AgCl��BaSO4��������ˮ����İ�ɫ���壩��
| ��������� | �жϺͻ�ѧ����ʽ | a | ������IΪ�ް�ζ�� | ����Ʒ�в��� ̼����� ̼����� �������ƣ��� |
b | �ɲ����������жϣ� | ����Ʒһ������ 笠� 笠� ���ӣ� |
c | �������Ϊ��ɫ�����������Ϊ������ | ����Ʒ�к��� ����� ����� ��д���ƣ����÷�Ӧ�Ļ�ѧ����ʽΪ��NH4��2SO4+Ba��NO3��2=BaSO4��+2NH4NO3 ��NH4��2SO4+Ba��NO3��2=BaSO4��+2NH4NO3 �� |
d | �������Ϊ���������������Ϊ��ɫ������ | ����Ʒ�к��� NH4Cl NH4Cl ��д��ѧʽ�����÷�Ӧ�Ļ�ѧ����ʽΪNH4Cl+AgNO3=AgCl��+NH4NO3 NH4Cl+AgNO3=AgCl��+NH4NO3 �� |
NH4NO3
NH4NO3
��д��ѧʽ����������������Ϊ����ζ���������������õ��ʿ���������
����
��ֻдһ�֣�����������1�����ݲ����������÷�����
��2��a�����ݳ�����̼������ֽ�����ʷ�����
b������笠����ӵļ��鷽��������
c��������������ӵļ��鷽��������
d�����������ӵļ��鷽��������
��3�����������ε����ʡ������ĵ��ʵ����������
��2��a�����ݳ�����̼������ֽ�����ʷ�����
b������笠����ӵļ��鷽��������
c��������������ӵļ��鷽��������
d�����������ӵļ��鷽��������
��3�����������ε����ʡ������ĵ��ʵ����������
����⣺��1���������ò��������Ŀ����ʹ��Ʒ����ܽ⣻
��2��a�����ڳ�����̼������ֽ�ų��������д̼�����ζ�����ԣ�������IΪ�ް�ζ������Ʒ�в���̼����泥�
b��������������ܷų����������ԣ�����Ʒ������ʯ�ң��а������ɣ�˵���˸���Ʒһ������笠����ӣ��õ���������Σ�
C��������������ᱵ��Һ��Ӧ���ɲ�����ϡ����İ�ɫ������˵���˸����������泥���Ӧ�ķ���ʽ����NH4��2SO4+Ba��NO3��2=BaSO4��+2NH4NO3��
d�������������������Һ��Ӧ���ɲ�����ϡ����İ�ɫ������˵���˸�������Ȼ�泥���Ӧ�ķ���ʽ��NH4Cl+AgNO3=AgCl��+NH4NO3��
��3�������������������õ��ʿ����� NH4NO3��������������Ϊ����ζ���������������õ��ʲ�����Σ����������أ�
�ʴ�Ϊ����1��ʹ��Ʒ����ܽ⣻��2��a��̼����泥�b��笠���c������泥���NH4��2SO4+Ba��NO3��2=BaSO4��+2NH4NO3��d��NH4Cl NH4Cl+AgNO3=AgCl��+NH4NO3����3��NH4NO3�����أ�
���е�ͼʾӦΪ��
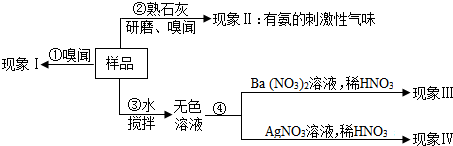
��2��a�����ڳ�����̼������ֽ�ų��������д̼�����ζ�����ԣ�������IΪ�ް�ζ������Ʒ�в���̼����泥�
b��������������ܷų����������ԣ�����Ʒ������ʯ�ң��а������ɣ�˵���˸���Ʒһ������笠����ӣ��õ���������Σ�
C��������������ᱵ��Һ��Ӧ���ɲ�����ϡ����İ�ɫ������˵���˸����������泥���Ӧ�ķ���ʽ����NH4��2SO4+Ba��NO3��2=BaSO4��+2NH4NO3��
d�������������������Һ��Ӧ���ɲ�����ϡ����İ�ɫ������˵���˸�������Ȼ�泥���Ӧ�ķ���ʽ��NH4Cl+AgNO3=AgCl��+NH4NO3��
��3�������������������õ��ʿ����� NH4NO3��������������Ϊ����ζ���������������õ��ʲ�����Σ����������أ�
�ʴ�Ϊ����1��ʹ��Ʒ����ܽ⣻��2��a��̼����泥�b��笠���c������泥���NH4��2SO4+Ba��NO3��2=BaSO4��+2NH4NO3��d��NH4Cl NH4Cl+AgNO3=AgCl��+NH4NO3����3��NH4NO3�����أ�
���е�ͼʾӦΪ��

�����������Ĺؼ��������̼����淋����ʡ�笠����ӡ���������ӡ������Ӽ��鷽����֪ʶ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ