题目内容
(18分)李兵发现:家里水壶用久之后,内壁常有一层不溶于水的水垢。为了解水垢的成因及成分,并寻找清洗水垢的方法,做了如下实验探究过程,请你参与:
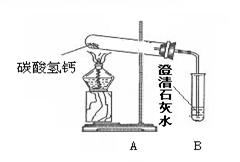
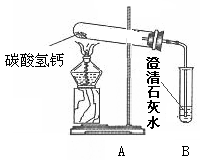
李兵查找资料后得知:自然界中的水中常溶有Ca(HCO3)2等可溶性物质。他提出[猜想Ⅰ]:水垢可能是烧开水时Ca(HCO3)2受热时发生分解产生。为了验证[猜想Ⅰ]是否正确,他做了如下图所示实验。

(1)加热一段时间后,发现试管口有水珠产生,B中出现白色浑浊,写出B中发生反应的化学方程式 。
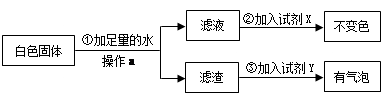
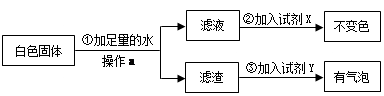
(2)实验结束后,试管中有白色固体存在,他将白色固体放入盛有足量水的烧杯中,充分搅拌,发现仍有白色固体。对白色固体的成分,李兵根据 定律提出[猜想Ⅱ]:认为白色固体可能是
a.CaCO3; b.Ca(OH)2; c.CaCO3和Ca(OH)2的混合物
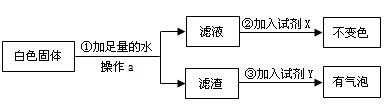
为了验证[猜想Ⅱ]中哪种情况是正确的,他又设计了如下实验方案:

试剂X是 ,试剂Y是 。则[猜想Ⅱ]中 (选填“a”、“b”或“c”)正确。
(3)据此,你认为上述有关水垢形成原因的[猜想Ⅰ]是 (选填“正确”或“错误“)。写出形成水垢的化学方程式 。
(4)刘豪同学觉得白色固体也可能是CaO,而李兵一下子否定了他的结论,理由是
。
(5)根据以上探究,李兵懂得了水垢的主要成分,他建议家庭清洗水垢时可选用 (选填序号)。
a.食醋 b.食盐水 c.水
(每小格各2分,共18分)
(1)Ca(OH)2 + CO2= CaCO3↓ + H2O
(2)质量守恒;无色酚酞;稀盐酸;c
(3)正确;Ca(HCO3)2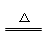 CaCO3↓ + H2O + CO2↑
CaCO3↓ + H2O + CO2↑
(4)氧化钙能与水反应生成氢氧化钙,使无色的酚酞试液变红色
(5)a
解析:本题是一道探究题,探究碳酸氢钙的性质,难度比较大