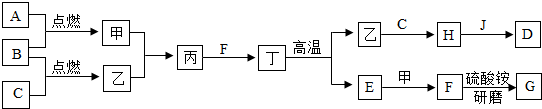
��Ŀ����
��1�����������׳� �� �� ���ڱ������Ϸ�һЩ�����������ƣ�¶���ڿ����У����Թ۲쵽������ȱ� ����һ��ʱ�䣬�����ֳ��ְ�ɫ��ĩ�����������仯��ԭ���� ����Ӧ�Ļ�ѧ����ʽΪ ��
��2�������������ơ��������Ƶȣ��������ƿ���ijЩ����ĸ�������磺����
�����壬�������ƿ�����ʯ����ˮ��Ӧ�Ƶã���ѧ����ʽΪ�� ��
��3����ͼ��ܷ����кͷ�Ӧ�������ճ������ũҵ�������й㷺��Ӧ�ã������᳧����ˮ�к�����������ʣ�������ʯ�ҽ��д�������Ӧ�Ļ�ѧ����ʽΪ�� ��
��4��5?12�봨������ֺ�������Ϊ����������Ⱥ�ڵ�����ˮ��ȫ���������߲���Ҫ����������ˮԴ���м�⣬��ÿɿ��Ŀ�ѧ���ݣ�������һƿˮ����Ҫ��ȡ�������ȣ�Ӧ��β����� ��
��2�������������ơ��������Ƶȣ��������ƿ���ijЩ����ĸ�������磺����
�����壬�������ƿ�����ʯ����ˮ��Ӧ�Ƶã���ѧ����ʽΪ��
��3����ͼ��ܷ����кͷ�Ӧ�������ճ������ũҵ�������й㷺��Ӧ�ã������᳧����ˮ�к�����������ʣ�������ʯ�ҽ��д�������Ӧ�Ļ�ѧ����ʽΪ��
��4��5?12�봨������ֺ�������Ϊ����������Ⱥ�ڵ�����ˮ��ȫ���������߲���Ҫ����������ˮԴ���м�⣬��ÿɿ��Ŀ�ѧ���ݣ�������һƿˮ����Ҫ��ȡ�������ȣ�Ӧ��β�����
���㣺����������Ժ���;,��Һ�����Ȳⶨ,��Ļ�ѧ����,��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ
ר�⣺�����ļ� ���ͨ��
��������1�������������Ƶ��׳��Լ����ʽ��н��
��2�������������ƹ�������ˮ�Լ������ƺ�ˮ��Ӧ�����������ƽ��н��
��3�������кͷ�Ӧ�Ǹ��ֽⷴӦ�е�һ��������ʽ���������ͨ�������ɷֶ������κ�ˮ�ķ�Ӧ���н��
��4���ⶨ��Һ������������Dz���PH��ֽ���м��飬��ʹ�÷����ǣ�˺��һ��pH��ֽ���ò�������ͷ�ι�ȡ��������Һ��������ֽ�ϣ���һ�������ɫ���Աȣ�����pHֵ���ɣ�
��2�������������ƹ�������ˮ�Լ������ƺ�ˮ��Ӧ�����������ƽ��н��
��3�������кͷ�Ӧ�Ǹ��ֽⷴӦ�е�һ��������ʽ���������ͨ�������ɷֶ������κ�ˮ�ķ�Ӧ���н��
��4���ⶨ��Һ������������Dz���PH��ֽ���м��飬��ʹ�÷����ǣ�˺��һ��pH��ֽ���ò�������ͷ�ι�ȡ��������Һ��������ֽ�ϣ���һ�������ɫ���Աȣ�����pHֵ���ɣ�
����⣺��1�����������׳��ռ�������ƣ�����ˮ���⣬���������̼��Ӧ����̼���ƺ�ˮ�����Ա����Ϊ���ռ�������ƣ���ʪ���������ƹ�������ˮ���⣬���������̼��Ӧ����̼���ƣ�2NaOH+CO2=Na2CO3+H2O��
��2�������������ơ��������ơ����������ȣ��������ƿ���ijЩ����ĸ���������Ը������Ժͼ������壬�����H2�����壻�������ƿ�����ʯ����ˮ��Ӧ�Ƶã��䷴Ӧ�Ļ�ѧ����ʽΪ CaO+H2O�TCa��OH��2�����H2��CaO+H2O�TCa��OH��2��
��3����������������ͨ�������ɷֶ���������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��H2SO4+Ca��OH��2=CaSO4+2H2O�����H2SO4+Ca��OH��2=CaSO4+2H2O��
��4��������ҺPH�ķ����ܶ࣬��ķ���������P��ֽ������Ҫ�IJ����ǣ�ȡ��һ��pH��ֽ���ò�������ͷ�ι�ȡ��������Һ��������ֽ�ϣ���һ�������ɫ���Աȣ�Ȼ�����pH��ֵ��
�ʴ�Ϊ��ȡ����ˮ�����ε�pH��ֽ�ϣ���pH��ֽ��ʾ����ɫ�����ɫ���Ƚϣ�����pH��
��2�������������ơ��������ơ����������ȣ��������ƿ���ijЩ����ĸ���������Ը������Ժͼ������壬�����H2�����壻�������ƿ�����ʯ����ˮ��Ӧ�Ƶã��䷴Ӧ�Ļ�ѧ����ʽΪ CaO+H2O�TCa��OH��2�����H2��CaO+H2O�TCa��OH��2��
��3����������������ͨ�������ɷֶ���������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��H2SO4+Ca��OH��2=CaSO4+2H2O�����H2SO4+Ca��OH��2=CaSO4+2H2O��
��4��������ҺPH�ķ����ܶ࣬��ķ���������P��ֽ������Ҫ�IJ����ǣ�ȡ��һ��pH��ֽ���ò�������ͷ�ι�ȡ��������Һ��������ֽ�ϣ���һ�������ɫ���Աȣ�Ȼ�����pH��ֵ��
�ʴ�Ϊ��ȡ����ˮ�����ε�pH��ֽ�ϣ���pH��ֽ��ʾ����ɫ�����ɫ���Ƚϣ�����pH��
���������⿼�����������Ƶ����ʣ���ɴ��⣬���������������Ƶ����ʽ��У�Ҫ��ͬѧ����ƽʱ��ѧϰ�м�ǿ����֪ʶ�Ĵ�����

��ϰ��ϵ�д�
�����Ŀ
�Ի�ѧʵ����Ϣ���з����ʹ����ǿ�ѧ̽������Ҫ���裬���ж�ʵ����Ϣ�ķ����ʹ�����ȷ���ǣ�������
| A����ȼ�ŵ�ľ�����뼯��ƿ��ľ��Ϩ��֤����ƿ�����Ƕ�����̼���� |
| B��ij�����м���ϡ���ᣬ�������壬֤����������һ������̼������� |
| C����ij��ɫ��Һ�е����̪��Һ����Һ����ɫ��˵����Һ������ |
| D����NaCl��Һ�м���NaCl���壬���ʵ������������ܲ��� |