��Ŀ����
��2013?���ݣ���1��������ʵ���ҳ����������ȡ���ռ�װ��ͼ��
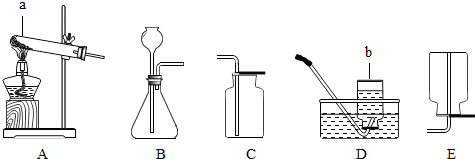
��д��������ŵ��������ƣ�a
��ʵ�����ù���������Һ��������̷�ĩ����ȡO2�����ж������̵�������
��2���������������������������Ħ��������Ҫ�ɷ���̼��ƣ���ͨ������ʵ��װ�òⶨ̼��Ƶ�������������������������ɷ��������Ӧ��
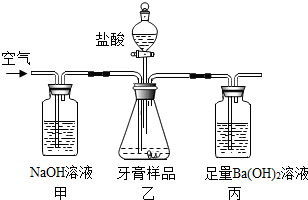
��̼�������
�����д�ʩ�в�����߲ⶨȷ�ȵ���
A����������εμ�����
B���ڼ��Ҽ�����װ��Ũ�����ϴ��ƿ��
C�����ұ�������װ�б���̼��������Һ��ϴ��ƿ��
D������װ�÷�Ӧ�������Լ���ͨ�����
������ȡ��������ƷΪag����װ���еij��������������Ϊbg������Ʒ��̼��Ƶ���������Ϊ
%���ú�a��b�Ĵ���ʽ��ʾ��
�������ⶨ��װ���г�������������ͨ���ⶨ��װ����ʵ��ǰ���������������̼��Ƶ������������ᵼ�²ⶨ�Ľ������ƫ�ߣ�ԭ����

��д��������ŵ��������ƣ�a
�Թ�
�Թ�
��b����ƿ
����ƿ
��ʵ�����ù���������Һ��������̷�ĩ����ȡO2�����ж������̵�������
������
������
��Ӧѡ�õ�����װ����B
B
�����ţ��ռ�O2����ѡ�õ�װ����E
E
�����ţ���2���������������������������Ħ��������Ҫ�ɷ���̼��ƣ���ͨ������ʵ��װ�òⶨ̼��Ƶ�������������������������ɷ��������Ӧ��

��̼�������
��
��
��ѡ��ᡱ������Ρ�����װ����̼��������ᷴӦ�Ļ�ѧ����ʽΪCaCO3+2HCl=CaCl2+H2O+CO2��
CaCO3+2HCl=CaCl2+H2O+CO2��
�����д�ʩ�в�����߲ⶨȷ�ȵ���
BC
BC
������ţ�A����������εμ�����
B���ڼ��Ҽ�����װ��Ũ�����ϴ��ƿ��
C�����ұ�������װ�б���̼��������Һ��ϴ��ƿ��
D������װ�÷�Ӧ�������Լ���ͨ�����
������ȡ��������ƷΪag����װ���еij��������������Ϊbg������Ʒ��̼��Ƶ���������Ϊ
| 10000b |
| 197a |
| 10000b |
| 197a |
�������ⶨ��װ���г�������������ͨ���ⶨ��װ����ʵ��ǰ���������������̼��Ƶ������������ᵼ�²ⶨ�Ľ������ƫ�ߣ�ԭ����
���е�ˮ�������Ȼ�������Ƚ���װ�ñ��У�ʹ��Һ����
���е�ˮ�������Ȼ�������Ƚ���װ�ñ��У�ʹ��Һ����
����������1���پݳ��������ش�
�ڶ��������ڹ���������Һ�ֽ���������ã��Ƿ�Ӧ�Ĵ��������ݷ�Ӧ��״̬�ͷ�Ӧ����ȷ������װ�ã����������ܶȺ��ܽ���ȷ���ռ�װ�ã�
��2�����ɽ������Ӻ����������ɵĻ��������Σ�����̼��ƺ����ᷴӦԭ����д����ʽ��
��A���μ�������죬�ᵼ�����ɵ�CO2������ȫ�����գ������ų�װ�ñ���
B���ڼ��Ҽ�����ʢ��Ũ�����ϴ��װ�ã�����ˮ�֣���Ӱ��CO2��
C�����ұ�֮������ʢ�б���̼��������Һ��ϴ��װ��Ba��OH��2����������CO2�е�HCl������Ӱ��CO2��
D����Ӧ�������Լ���ͨ�������ʹ���ɵĶ�����̼��ȫ���ų���Ӱ��CO2��
�۸���̼�ᱵ�������������̼�����������ݶ�����̼��������̼��Ƶ���������������������������÷���ʽ�ҳ����ʼ��������ϵ���ù�ϵʽ��⣻
��B�е�ˮ�������Ȼ�������Ƚ���װ��C�л�ʹ��Һ�������ӣ�
�ڶ��������ڹ���������Һ�ֽ���������ã��Ƿ�Ӧ�Ĵ��������ݷ�Ӧ��״̬�ͷ�Ӧ����ȷ������װ�ã����������ܶȺ��ܽ���ȷ���ռ�װ�ã�
��2�����ɽ������Ӻ����������ɵĻ��������Σ�����̼��ƺ����ᷴӦԭ����д����ʽ��
��A���μ�������죬�ᵼ�����ɵ�CO2������ȫ�����գ������ų�װ�ñ���
B���ڼ��Ҽ�����ʢ��Ũ�����ϴ��װ�ã�����ˮ�֣���Ӱ��CO2��
C�����ұ�֮������ʢ�б���̼��������Һ��ϴ��װ��Ba��OH��2����������CO2�е�HCl������Ӱ��CO2��
D����Ӧ�������Լ���ͨ�������ʹ���ɵĶ�����̼��ȫ���ų���Ӱ��CO2��
�۸���̼�ᱵ�������������̼�����������ݶ�����̼��������̼��Ƶ���������������������������÷���ʽ�ҳ����ʼ��������ϵ���ù�ϵʽ��⣻
��B�е�ˮ�������Ȼ�������Ƚ���װ��C�л�ʹ��Һ�������ӣ�
����⣺��1���ٱ�������ֱ����Թܺͼ���ƿ��
�ڶ��������ڹ���������Һ�ֽ���������ã��Ƿ�Ӧ�Ĵ������ܼӿ��������ķֽ����ʣ��÷�Ӧ������ȣ����ڹ�Һ�����ͣ���ѡ����װ��B���������ܶȱȿ������Ҳ�������ˮ���ʿ��������ſ���������ˮ���ռ����������������ſ������ռ���
��2����̼����ɸ����Ӻ�̼������ӹ��ɣ������Σ�̼��ƺ����ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼������ʽ�ǣ�CaCO3+2HCl=CaCl2+H2O+CO2����
��A���μ�������죬�ᵼ�����ɵ�CO2������ȫ�����գ������ų�װ�ñ������Ի�������εμ����ᣬ��ʹ���ɵ�CO2��ȫ�����գ����ʵ��IJⶨȷ�ȣ�
B���ڼ��Ҽ�����ʢ��Ũ�����ϴ��װ�ã�����ˮ�֣���Ӱ��CO2����Ӱ��ʵ��IJⶨȷ�ȣ�
C�����ұ�֮������ʢ�б���̼��������Һ��ϴ��װ��Ba��OH��2����������CO2�е�HCl������Ӱ��CO2���ʲ�Ӱ��ʵ��IJⶨȷ�ȣ�
D����Ӧ�������Լ���ͨ�������ʹ���ɵĶ�����̼��ȫ���ų���Ӱ��CO2�����������ʵ��IJⶨȷ�ȣ�
�۸��ݷ���ʽCaCO3+2HCl�TCaCl2+H2O+CO2����CO2+Ba��OH��2�TBaCO3��+H2O��֪���ʼ�Ĺ�ϵ���£���̼��Ƶ�����Ϊx
CaCO3��CO2��BaCO3��
100 197
x bg
=
x=
g
����̼��Ƶ����������ǣ�
��100%=
%
�����е�ˮ�������Ȼ�������Ƚ���װ�ñ��У����²ⶨ������̼������ƫ�ⶨ��̼��Ƶ�����ƫ��̼��Ƶ���������ƫ�ߣ�
�ʴ�Ϊ����1�����Թܣ�����ƿ���ڴ����ã�B��E��
��2������CaCO3+2HCl=CaCl2+H2O+CO2������BC����
�������е�ˮ�������Ȼ�������Ƚ���װ�ñ��У�ʹ��Һ���أ�
�ڶ��������ڹ���������Һ�ֽ���������ã��Ƿ�Ӧ�Ĵ������ܼӿ��������ķֽ����ʣ��÷�Ӧ������ȣ����ڹ�Һ�����ͣ���ѡ����װ��B���������ܶȱȿ������Ҳ�������ˮ���ʿ��������ſ���������ˮ���ռ����������������ſ������ռ���
��2����̼����ɸ����Ӻ�̼������ӹ��ɣ������Σ�̼��ƺ����ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼������ʽ�ǣ�CaCO3+2HCl=CaCl2+H2O+CO2����
��A���μ�������죬�ᵼ�����ɵ�CO2������ȫ�����գ������ų�װ�ñ������Ի�������εμ����ᣬ��ʹ���ɵ�CO2��ȫ�����գ����ʵ��IJⶨȷ�ȣ�
B���ڼ��Ҽ�����ʢ��Ũ�����ϴ��װ�ã�����ˮ�֣���Ӱ��CO2����Ӱ��ʵ��IJⶨȷ�ȣ�
C�����ұ�֮������ʢ�б���̼��������Һ��ϴ��װ��Ba��OH��2����������CO2�е�HCl������Ӱ��CO2���ʲ�Ӱ��ʵ��IJⶨȷ�ȣ�
D����Ӧ�������Լ���ͨ�������ʹ���ɵĶ�����̼��ȫ���ų���Ӱ��CO2�����������ʵ��IJⶨȷ�ȣ�
�۸��ݷ���ʽCaCO3+2HCl�TCaCl2+H2O+CO2����CO2+Ba��OH��2�TBaCO3��+H2O��֪���ʼ�Ĺ�ϵ���£���̼��Ƶ�����Ϊx
CaCO3��CO2��BaCO3��
100 197
x bg
| 100 |
| x |
| 197 |
| bg |
x=
| 100b |
| 197 |
����̼��Ƶ����������ǣ�
| ||
| ag |
| 10000b |
| 197a |
�����е�ˮ�������Ȼ�������Ƚ���װ�ñ��У����²ⶨ������̼������ƫ�ⶨ��̼��Ƶ�����ƫ��̼��Ƶ���������ƫ�ߣ�
�ʴ�Ϊ����1�����Թܣ�����ƿ���ڴ����ã�B��E��
��2������CaCO3+2HCl=CaCl2+H2O+CO2������BC����
| 10000b |
| 197a |
�����������ܺܺõĿ���ѧ����֪ʶ�����պ�Ӧ�ã������������������������ѶȽϴ�Ҫ�����֪ʶ��Ͼ����龰��ϸ�������

��ϰ��ϵ�д�
�����Ŀ