��Ŀ����
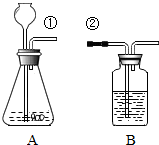 ij��ȤС�鰴��ͼ��ʾװ����ȡ������̼�������������̼�����ʣ�
ij��ȤС�鰴��ͼ��ʾװ����ȡ������̼�������������̼�����ʣ���1������װ��B��֤��������̼����ˮ��Ӧ����̼�ᣬB�г�������ˮ�⣬��Ӧ������Լ���
��2�����Ǵ�����һ�Ž���ά���ֳ��Ѽ���һЩ��ǽ�ң�ͨ���������ϵ�֪���Ž�����ǽ�ҵ���Ҫ�ɷ���̼��ƣ����dz�ȡ��12.0g��ǽ�ҷ����ձ��У�����������ϡ���ᣨ�������ʲ����뷴Ӧ��������H2O��HCl���ݳ�������Ӧ��ʼʱ���ձ�����ʢ���ʵ�������Ϊ300.0g��ʵ�����ݼ�¼���£�
| ��Ӧʱ��/min | 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 |
| �ձ�����ʢ����������/g | 300.0 | 299.0 | 298.0 | 297.2 | 296.5 | 296.0 | 295.7 | 295.6 | m | 295.6 |
�ڷ�Ӧ���ɶ�����̼��������Ϊ
�۾�ǽ����̼��Ƶ�����������д��������̣���
��������1����Ҫ�漰�˶�����̼����ˮʱ�����в��ֶ�����̼��ˮ��Ӧ����̼�ᣬʯ���Լ�Һ��ʹ�������ʱ�����ʵĿ��飻
��2���ٳ�����ñ��е���Ϣ14���18��ʱ����ֵ���䣬�������Ʋ�M����ֵӦ��ͬ���ڸ��������غ㶨�ɲ��ѵó����ٵ�������Ϊ���ɶ�����̼�������۳�����û�ѧ����ʽ�������غ㶨�ɽ��мĻ�ѧ�����һ���⣬ͬʱ��֪������������
��2���ٳ�����ñ��е���Ϣ14���18��ʱ����ֵ���䣬�������Ʋ�M����ֵӦ��ͬ���ڸ��������غ㶨�ɲ��ѵó����ٵ�������Ϊ���ɶ�����̼�������۳�����û�ѧ����ʽ�������غ㶨�ɽ��мĻ�ѧ�����һ���⣬ͬʱ��֪������������
����⣺��1��������̼����ˮ��Ӧ����̼�ᣬ����������ʹ��ɫʯ���죬���Է�Ӧ�Ļ�ѧ����ʽΪ��CO2+H2O=H2CO3��
��2���ٽ�ϱ����е���Ϣ���ڷ�Ӧ14���18��ʱ���ձ���ʢ�е�����Ϊ295.6g������16��ʱ����һ��MΪ295.6g��
�ڸ���������Ϣ���ʲ����뷴Ӧ��������H2O��HCl���ݳ����ٽ�������غ㶨�ɼ��ٵ�����Ϊ300.0g-295.6g=4.4g��һ��Ϊ���ɵĶ�����̼��������
��Ҫ���ǽ����̼��Ƶ�����������������������غ㶨�ɽ�ϻ�ѧ��Ӧ����ʽ�������̼��Ƶ����������Կ���12.0g��ǽ�Һ�̼��Ƶ�����Ϊx������
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 4.4g
=
x=10g
���Ծ�ǽ����̼��Ƶ���������=
��100%=83.3%
�ʴ�Ϊ����1��ʯ����Һ��ʯ����Һ���ϱ�죻CO2+H2O=H2CO3��
��2����295.6��300.0g-295.6g=4.4g
�۽⣺��12.0g��ǽ�Һ�̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 4.4g
=
x=10g
���Ծ�ǽ����̼��Ƶ���������=
��100%=83.3%
�𣺴˾�ǽ����̼��Ƶ���������83.3%
��2���ٽ�ϱ����е���Ϣ���ڷ�Ӧ14���18��ʱ���ձ���ʢ�е�����Ϊ295.6g������16��ʱ����һ��MΪ295.6g��
�ڸ���������Ϣ���ʲ����뷴Ӧ��������H2O��HCl���ݳ����ٽ�������غ㶨�ɼ��ٵ�����Ϊ300.0g-295.6g=4.4g��һ��Ϊ���ɵĶ�����̼��������
��Ҫ���ǽ����̼��Ƶ�����������������������غ㶨�ɽ�ϻ�ѧ��Ӧ����ʽ�������̼��Ƶ����������Կ���12.0g��ǽ�Һ�̼��Ƶ�����Ϊx������
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 4.4g
| 100 |
| 44 |
| X |
| 4.4g |
x=10g
���Ծ�ǽ����̼��Ƶ���������=
| 10g |
| 12g |
�ʴ�Ϊ����1��ʯ����Һ��ʯ����Һ���ϱ�죻CO2+H2O=H2CO3��
��2����295.6��300.0g-295.6g=4.4g
�۽⣺��12.0g��ǽ�Һ�̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 4.4g
| 100 |
| 44 |
| X |
| 4.4g |
x=10g
���Ծ�ǽ����̼��Ƶ���������=
| 10g |
| 12g |
�𣺴˾�ǽ����̼��Ƶ���������83.3%
������������һ�����ڶ�����̼��ʵ������ȡ��������̼��ѧ���ʵļ��飬���������غ㶨�ɽ��м���Ļ�ѧ�⣮���ʱӦ���������Ŀ�и����ͼ����Ϣ���з��������������غ㶨��ȷ�����ٵ�����Ϊ������̼��ȷ��д��ѧ����ʽ�ǽ�����Ĺؼ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ij��ȤС�鰴��ͼ��ʾװ����ȡ������̼�������������̼�����ʣ�
ij��ȤС�鰴��ͼ��ʾװ����ȡ������̼�������������̼�����ʣ�
��1������װ��B��֤��������̼����ˮ��Ӧ����̼�ᣬB�г�������ˮ�⣬��Ӧ������Լ���______�����١��ڵ��ܿ�����ʱ��B�п�����ʵ��������______����Ӧ�Ļ�ѧ����ʽ��______
��2�����Ǵ�����һ�Ž���ά���ֳ��Ѽ���һЩ��ǽ�ң�ͨ���������ϵ�֪���Ž�����ǽ�ҵ���Ҫ�ɷ���̼��ƣ����dz�ȡ��12.0g��ǽ�ҷ����ձ��У�����������ϡ���ᣨ�������ʲ����뷴Ӧ��������H2O��HCl���ݳ�������Ӧ��ʼʱ���ձ�����ʢ���ʵ�������Ϊ300.0g��ʵ�����ݼ�¼���£�
| ��Ӧʱ��/min | 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 |
| �ձ�����ʢ����������/g | 300.0 | 299.0 | 298.0 | 297.2 | 296.5 | 296.0 | 295.7 | 295.6 | m | 295.6 |
�ڷ�Ӧ���ɶ�����̼��������Ϊ______���г���ʽ����
�۾�ǽ����̼��Ƶ�����������д��������̣�����
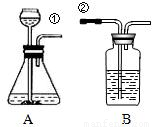
ij��ȤС�鰴��ͼ��ʾװ����ȡ������̼�������������̼�����ʣ�
��1������װ��B��֤��������̼����ˮ��Ӧ����̼�ᣬB�г�������ˮ�⣬��Ӧ������Լ���______�����١��ڵ��ܿ�����ʱ��B�п�����ʵ��������______����Ӧ�Ļ�ѧ����ʽ��______
��2�����Ǵ�����һ�Ž���ά���ֳ��Ѽ���һЩ��ǽ�ң�ͨ���������ϵ�֪���Ž�����ǽ�ҵ���Ҫ�ɷ���̼��ƣ����dz�ȡ��12.0g��ǽ�ҷ����ձ��У�����������ϡ���ᣨ�������ʲ����뷴Ӧ��������H2O��HCl���ݳ�������Ӧ��ʼʱ���ձ�����ʢ���ʵ�������Ϊ300.0g��ʵ�����ݼ�¼���£�
���Ը�С�����û�м�¼��Ӧʱ��Ϊ16minʱ������M���ɱ��������Ʋ⣬M=______g��
�ڷ�Ӧ���ɶ�����̼��������Ϊ______���г���ʽ����
�۾�ǽ����̼��Ƶ�����������д��������̣���______��
��1������װ��B��֤��������̼����ˮ��Ӧ����̼�ᣬB�г�������ˮ�⣬��Ӧ������Լ���______�����١��ڵ��ܿ�����ʱ��B�п�����ʵ��������______����Ӧ�Ļ�ѧ����ʽ��______
��2�����Ǵ�����һ�Ž���ά���ֳ��Ѽ���һЩ��ǽ�ң�ͨ���������ϵ�֪���Ž�����ǽ�ҵ���Ҫ�ɷ���̼��ƣ����dz�ȡ��12.0g��ǽ�ҷ����ձ��У�����������ϡ���ᣨ�������ʲ����뷴Ӧ��������H2O��HCl���ݳ�������Ӧ��ʼʱ���ձ�����ʢ���ʵ�������Ϊ300.0g��ʵ�����ݼ�¼���£�
| ��Ӧʱ��/min | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | |
| �ձ�����ʢ����������/g | 300.0 | 299.0 | 298.0 | 297.2 | 296.5 | 296.0 | 295.7 | 295.6 | m | 295.6 |
�ڷ�Ӧ���ɶ�����̼��������Ϊ______���г���ʽ����
�۾�ǽ����̼��Ƶ�����������д��������̣���______��

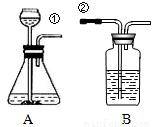
ij��ȤС�鰴��ͼ��ʾװ����ȡ������̼�������������̼�����ʣ�
��1������װ��B��֤��������̼����ˮ��Ӧ����̼�ᣬB�г�������ˮ�⣬��Ӧ������Լ���______�����١��ڵ��ܿ�����ʱ��B�п�����ʵ��������______����Ӧ�Ļ�ѧ����ʽ��______
��2�����Ǵ�����һ�Ž���ά���ֳ��Ѽ���һЩ��ǽ�ң�ͨ���������ϵ�֪���Ž�����ǽ�ҵ���Ҫ�ɷ���̼��ƣ����dz�ȡ��12.0g��ǽ�ҷ����ձ��У�����������ϡ���ᣨ�������ʲ����뷴Ӧ��������H2O��HCl���ݳ�������Ӧ��ʼʱ���ձ�����ʢ���ʵ�������Ϊ300.0g��ʵ�����ݼ�¼���£�
���Ը�С�����û�м�¼��Ӧʱ��Ϊ16minʱ������M���ɱ��������Ʋ⣬M=______g��
�ڷ�Ӧ���ɶ�����̼��������Ϊ______���г���ʽ����
�۾�ǽ����̼��Ƶ�����������д��������̣���______��
��1������װ��B��֤��������̼����ˮ��Ӧ����̼�ᣬB�г�������ˮ�⣬��Ӧ������Լ���______�����١��ڵ��ܿ�����ʱ��B�п�����ʵ��������______����Ӧ�Ļ�ѧ����ʽ��______
��2�����Ǵ�����һ�Ž���ά���ֳ��Ѽ���һЩ��ǽ�ң�ͨ���������ϵ�֪���Ž�����ǽ�ҵ���Ҫ�ɷ���̼��ƣ����dz�ȡ��12.0g��ǽ�ҷ����ձ��У�����������ϡ���ᣨ�������ʲ����뷴Ӧ��������H2O��HCl���ݳ�������Ӧ��ʼʱ���ձ�����ʢ���ʵ�������Ϊ300.0g��ʵ�����ݼ�¼���£�
| ��Ӧʱ��/min | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | |
| �ձ�����ʢ����������/g | 300.0 | 299.0 | 298.0 | 297.2 | 296.5 | 296.0 | 295.7 | 295.6 | m | 295.6 |
�ڷ�Ӧ���ɶ�����̼��������Ϊ______���г���ʽ����
�۾�ǽ����̼��Ƶ�����������д��������̣���______��

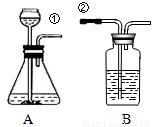
ij��ȤС�鰴��ͼ��ʾװ����ȡ������̼�������������̼�����ʣ�
��1������װ��B��֤��������̼����ˮ��Ӧ����̼�ᣬB�г�������ˮ�⣬��Ӧ������Լ���______�����١��ڵ��ܿ�����ʱ��B�п�����ʵ��������______����Ӧ�Ļ�ѧ����ʽ��______
��2�����Ǵ�����һ�Ž���ά���ֳ��Ѽ���һЩ��ǽ�ң�ͨ���������ϵ�֪���Ž�����ǽ�ҵ���Ҫ�ɷ���̼��ƣ����dz�ȡ��12.0g��ǽ�ҷ����ձ��У�����������ϡ���ᣨ�������ʲ����뷴Ӧ��������H2O��HCl���ݳ�������Ӧ��ʼʱ���ձ�����ʢ���ʵ�������Ϊ300.0g��ʵ�����ݼ�¼���£�
���Ը�С�����û�м�¼��Ӧʱ��Ϊ16minʱ������M���ɱ��������Ʋ⣬M=______g��
�ڷ�Ӧ���ɶ�����̼��������Ϊ______���г���ʽ����
�۾�ǽ����̼��Ƶ�����������д��������̣���______��
��1������װ��B��֤��������̼����ˮ��Ӧ����̼�ᣬB�г�������ˮ�⣬��Ӧ������Լ���______�����١��ڵ��ܿ�����ʱ��B�п�����ʵ��������______����Ӧ�Ļ�ѧ����ʽ��______
��2�����Ǵ�����һ�Ž���ά���ֳ��Ѽ���һЩ��ǽ�ң�ͨ���������ϵ�֪���Ž�����ǽ�ҵ���Ҫ�ɷ���̼��ƣ����dz�ȡ��12.0g��ǽ�ҷ����ձ��У�����������ϡ���ᣨ�������ʲ����뷴Ӧ��������H2O��HCl���ݳ�������Ӧ��ʼʱ���ձ�����ʢ���ʵ�������Ϊ300.0g��ʵ�����ݼ�¼���£�
| ��Ӧʱ��/min | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | |
| �ձ�����ʢ����������/g | 300.0 | 299.0 | 298.0 | 297.2 | 296.5 | 296.0 | 295.7 | 295.6 | m | 295.6 |
�ڷ�Ӧ���ɶ�����̼��������Ϊ______���г���ʽ����
�۾�ǽ����̼��Ƶ�����������д��������̣���______��
