��Ŀ����
 С��ͬѧΪ�о�����������Ⱥ��������ʵ���ɣ���������ʵ�飺ȡ2.50g��������
С��ͬѧΪ�о�����������Ⱥ��������ʵ���ɣ���������ʵ�飺ȡ2.50g��������
��CuSO4?XH2O������ʹ��ֽ⣬���Ƴ�������������¶ȵı仯��ϵͼ����ͼ��
t1��ʱ�ù�����ȫʧȥ�ᾧˮ����ѧ����ʽΪ��CuSO4?XH2O  CuSO4+XH2O����
CuSO4+XH2O����
��1�����㵨��������X��ֵ����ע��CuSO4?XH2O����Է��������ɱ�ʾΪ��160+18X��
��2�����µ�t2�棬���������Ǻ�ɫ���ʣ�Ԥ���仯ѧʽΪ______��m=______g����д����m�Ĺ��̣�
�⣺��1����ͼʾ���ݺ͡���t1��ʱ�ù�����ȫʧȥ�ᾧˮ����֪���ᾧˮ������Ϊ��2.50g-1.60g=0.90g
CuSO4?xH2O CuSO4+xH2O����
CuSO4+xH2O����
160+18x 18x
2.50g 0.90g
��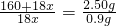
��160+18x=50x��
��x=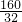 =5��
=5��
��2������ԭ���غ㣬��ɫ������һ������ͭ����ѧʽCuO��
��Ϊ��ɫ������CuO����CuԪ���غ�ã�m��Cu��=1.60g��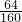 =0.64g��
=0.64g��
����m��CuO��=0.64g�� =0.80g��
=0.80g��
�ʴ�Ϊ����1��5����2��CuO�� 0.8g
��������1����ͼʾ���ݺ͡���t1��ʱ�ù�����ȫʧȥ�ᾧˮ����֪���ᾧˮ������=2.50g-1.60g��Ȼ����ݵ����������ȷֽ�Ļ�ѧ����ʽ�͵�����ᾧˮ���������г�����ʽ���Ϳɼ��������������x��ֵ��
��2����Ϊ���µ�t2��ʱ������ͭ���ȼ����ֽ⣬���������غ㶨�ɣ�����������һ����Cu��Ԥ���ɫ���ʣ������m��
������������Ҫ����ѧ��������ѧ��ѧ֪ʶ�ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������
CuSO4?xH2O
 CuSO4+xH2O����
CuSO4+xH2O����160+18x 18x
2.50g 0.90g
��
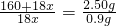
��160+18x=50x��
��x=
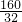 =5��
=5����2������ԭ���غ㣬��ɫ������һ������ͭ����ѧʽCuO��
��Ϊ��ɫ������CuO����CuԪ���غ�ã�m��Cu��=1.60g��
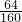 =0.64g��
=0.64g������m��CuO��=0.64g��
 =0.80g��
=0.80g���ʴ�Ϊ����1��5����2��CuO�� 0.8g
��������1����ͼʾ���ݺ͡���t1��ʱ�ù�����ȫʧȥ�ᾧˮ����֪���ᾧˮ������=2.50g-1.60g��Ȼ����ݵ����������ȷֽ�Ļ�ѧ����ʽ�͵�����ᾧˮ���������г�����ʽ���Ϳɼ��������������x��ֵ��
��2����Ϊ���µ�t2��ʱ������ͭ���ȼ����ֽ⣬���������غ㶨�ɣ�����������һ����Cu��Ԥ���ɫ���ʣ������m��
������������Ҫ����ѧ��������ѧ��ѧ֪ʶ�ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��1������5�£����и�ѧУ������ʵ��������飮С��ͬѧ���е�ʵ�鿼���ǡ���ϡ��������������ƣ�NaOH����Һ��̼���ƣ�Na2CO3����Һ�����ⶨ̼������Һ��pH����
��С��ͬѧ��ʵ���¼���±����벹��������
| �������� | �����¼ | ��Ӧ�Ļ�ѧ����ʽ |
| ȡ��֧�Թܣ��ֱ����Թ��м���2mL��Ʒ1��2���������е������� | ��Ʒ1������������ | ______ |
| ��Ʒ2����Һ�г������� | ______ |
����pH��ֽ�ⶨ̼������Һ�����ȣ�������������Ҫ���裺______��
��2����ʵ��̨������ƿδ����ǩ����Һ����֪�ֱ���̼������Һ������������Һ��ϡ���ᣮΪ������������Һ������ʦָ���£���ȤС���ͬѧ����������Һ��A��B��C���б�ţ�Ȼ��ֱ��ȡ������Ϊ��Ʒ���뵽��֧�Թ��У���������ͼ��ʾ��̽�����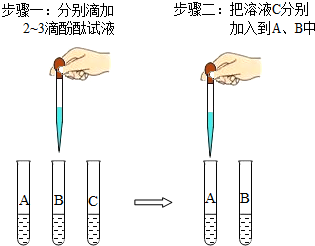
����һ�У�C����������A��B��Һ����ɫ��ɺ�ɫ��
������У�A��B��Һ����ɫ�ɺ�ɫ�����ɫ����B��Һ��������ð����
�ٸ�������ʵ�������֪��B��C��Һ�ֱ���______��______��
��ijС��ͬѧ�ڽ��в���һʵ��ʱ�����쳣������A��Һ�м����̪��Һʱ����Һ��ɫ�ȱ�ɺ�ɫ����Ѹ�ٱ����ɫ����ʦָ��������Ϊ��ҺŨ�ȹ�����ɵģ����ţ���ʦ�����Թܵ���Һ�м����������ᣬ�۲쵽A��Һ����ɫ�ֱ�ɺ�ɫ����ʱ��Һ�к��е�������Ҫ��______����̪���⣩��
�ס�����ͬѧ���˽�����������Һ������ԣ��������������о���
| ʵ����� | ����� | |
| ��ͬѧ | ȡpH��ֽ���ڲ���Ƭ�ϣ��ò�����պȡ����������Һմ��pH��ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ� | pH��7 |
| ��ͬѧ | ��pH��ֱֽ�ӽ�������������Һ�У�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ� | pH��7 |
��2��������λͬѧ��ʵ�����������Ϊ______ͬѧ�������淶��Ϊʲô����PH��ֽ�ⶨ��Һ����Ե���ȷ���������ǣ�ȡPH ��ֽ���ڲ���Ƭ�ϣ��ò�����պȡ������Һ����PH ��ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ�����ͬѧ��PH ��ֱֽ�ӽ�������������Һ������������Ⱦ�Լ��������Ǵ���ģ�
��3�����ʵ����û��pH��ֽ����Ҫһ�βⶨδ֪��Һ������ԣ���ѡ��______��Һ������ʵ�飬���Լ���______ɫ���______ɫ����ȷ�����Լ��ԣ���ʵ��������ƿʧȥ��ǩ������������Һ��һƿŨ��Һ��һƿϡ��Һ______�����ܻ��ܣ��ø��Լ��ⶨ��Ũϡ��ԭ����______��
 ______+6H2O��
______+6H2O��

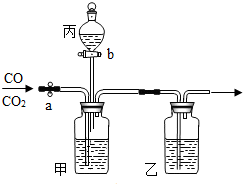 ��ʵ��������ͼ��ʾװ�ý���CO��CO2�ķ�������ֻ��ϡ���ᡢŨ���ᡢʯ��ˮ����Ҫ����д���пհ�
��ʵ��������ͼ��ʾװ�ý���CO��CO2�ķ�������ֻ��ϡ���ᡢŨ���ᡢʯ��ˮ����Ҫ����д���пհ�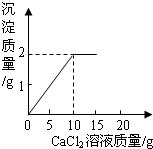 ��֪CaCl2+Na2CO3=CaCO3��+2NaCl����15g CaCl2��Һ��μ��뵽20g Na2CO3��Һ�У���������ɳ��������������CaCl2��Һ��������ϵ��ͼ��ʾ��������Ӧ������ʹ��ˣ�������Һ��õ�����������Ƕ��٣�
��֪CaCl2+Na2CO3=CaCO3��+2NaCl����15g CaCl2��Һ��μ��뵽20g Na2CO3��Һ�У���������ɳ��������������CaCl2��Һ��������ϵ��ͼ��ʾ��������Ӧ������ʹ��ˣ�������Һ��õ�����������Ƕ��٣�